|
Polyimide
Polyimide (sometimes abbreviated PI) is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-oxydianiline.Wright, Walter W. and Hallden-Abberton, Michael (2002) "Polyimides" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. History The first polyimide was discovered in 1908 by Bogart and Renshaw. They found that 4-amino phthalic anhydride does not melt when heated but does release water upon the formation of a high molecular weight polyimide. The first semialiphatic polyimide was prepared by Edward and Robinson by melt fusion of diamines and tetra acids or diamines and diacids / diester. However, the first polyimide of significan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyimide
Polyimide (sometimes abbreviated PI) is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-oxydianiline.Wright, Walter W. and Hallden-Abberton, Michael (2002) "Polyimides" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. History The first polyimide was discovered in 1908 by Bogart and Renshaw. They found that 4-amino phthalic anhydride does not melt when heated but does release water upon the formation of a high molecular weight polyimide. The first semialiphatic polyimide was prepared by Edward and Robinson by melt fusion of diamines and tetra acids or diamines and diacids / diester. However, the first polyimide of significan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyimide Formation (schematic) V1
Polyimide (sometimes abbreviated PI) is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-oxydianiline.Wright, Walter W. and Hallden-Abberton, Michael (2002) "Polyimides" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. History The first polyimide was discovered in 1908 by Bogart and Renshaw. They found that 4-amino phthalic anhydride does not melt when heated but does release water upon the formation of a high molecular weight polyimide. The first semialiphatic polyimide was prepared by Edward and Robinson by melt fusion of diamines and tetra acids or diamines and diacids / diester. However, the first polyimide of significan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoset Polymer Matrix
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle. They were first used after World War II, and continuing research has led to an increased range of thermoset resins, polymers or plastics, as well as engineering grade thermoplastics. They were all developed for use in the manufacture of polymer composites with enhanced and longer-term service capabilities. Thermoset polymer matrix technologies also find use in a wide diversity of non-structural industrial applications. The foremost types of thermosetting polymers used in structural composites are benzoxazine resins, bis-maleimide resins (BMI), cyanate ester resins, epoxy (epoxide) resins, phenolic (PF) resins, unsaturated polyester ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kapton
Structure of poly-oxydiphenylene-pyromellitimide Kapton insulating pads for mounting electronic parts on a heat sink Kapton is a polyimide film used in flexible printed circuits ( flexible electronics) and space blankets, which are used on spacecraft, satellites, and various space instruments. Invented by the DuPont Corporation in the 1960s, Kapton remains stable (in isolation) across a wide range of temperatures, from . History Kapton was invented by the DuPont Corporation in the 1960s. The name ''Kapton'' is a registered trademark of E. I. du Pont de Nemours and Company. Chemistry and variants Kapton synthesis is an example of the use of a dianhydride in step polymerization. The intermediate polymer, known as a ''poly(amic acid)'', is soluble because of strong hydrogen bonds to the polar solvents usually employed in the reaction. The ring closure is carried out at high temperatures of . The chemical name for Kapton K and HN is ''poly (4,4'-oxydiphenylene-pyromelli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imide
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand. Nomenclature Most imides are cyclic compounds derived from dicarboxylic acids, and their names reflect the parent acid. Examples are succinimide, derived from succinic acid, and phthalimide, derived from phthalic acid. For imides derived from amines (as opposed to ammonia), the ''N''-substituent is indicated by a prefix. For example, N-ethylsuccinimide is derived from succinic acid and ethylamine. Isoimides are isomeric with normal imides and have the formula RC(O)OC(NR′)R″. They are often intermediates that convert to the more sym ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4,4'-Diaminodiphenyl Ether
4,4′-Oxydianiline (ODA) is an organic compound with the formula O( C6 H4 NH2)2. It is an ether derivative of aniline. This colourless solid is a useful monomer and cross-linking agent for polymers, especially the polyimides, such as Kapton. Uses 4,4′-Oxydianiline is used in the production of a wide variety of polymer resins. The primary use lies in the production of polyimide and poly(ester)imide resins. These resins are used for their temperature-resistant properties and are utilized in products including wire enamels, coatings, film, adhesives, insulating varnishes, coated fabrics, flame-retardant fibers, oil sealants and retainers, insulation for cables and printed circuits, and laminates and composite for aerospace vehicles. Other applications of 4,4′-oxydianiline include the production of poly(amide)imide resins (which are used in the manufacture of heat-resistant wire enamels and coatings), as an intermediate in the manufacture of epoxy resins and adhesives, and in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyromellitic Dianhydride
Pyromellitic dianhydride (PMDA) is an organic compound with the formula C6H2(C2O3)2. It is the double carboxylic acid anhydride that is used in the preparation of polyimide polymers such as Kapton. It is a white, hygroscopic solid. It forms a hydrate. Preparation It is prepared by gas-phase oxidation of 1,2,4,5-tetramethylbenzene (or related tetrasubstituted benzene derivatives). An idealized equation is: :C6H2(CH3)4 + 6 O2 → C6H2(C2O3)2 + 6 H2O In the laboratory, it can be prepared by dehydration of pyromellitic acid using acetic anhydride. Reactions PMDA is an electron-acceptor, forming a variety of charge-transfer complex In chemistry, a charge-transfer (CT) complex or electron-donor-acceptor complex describes a type of supramolecular assembly of two or more molecules or ions. The assembly consists of two molecules that self-attract through electrostatic forc ...es. It reacts with amines to diimides, C6H2 CO)2NRsub>2 which also have acceptor propertie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4,4'-Oxydianiline
4,4′-Oxydianiline (ODA) is an organic compound with the formula O( C6 H4 NH2)2. It is an ether derivative of aniline. This colourless solid is a useful monomer and cross-linking agent for polymers, especially the polyimides, such as Kapton. Uses 4,4′-Oxydianiline is used in the production of a wide variety of polymer resins. The primary use lies in the production of polyimide and poly(ester)imide resins. These resins are used for their temperature-resistant properties and are utilized in products including wire enamels, coatings, film, adhesives, insulating varnishes, coated fabrics, flame-retardant fibers, oil sealants and retainers, insulation for cables and printed circuits, and laminates and composite for aerospace vehicles. Other applications of 4,4′-oxydianiline include the production of poly(amide)imide resins (which are used in the manufacture of heat-resistant wire enamels and coatings), as an intermediate in the manufacture of epoxy resins and adhesives, and in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamine
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to primary diamines, as those are the most reactive. In terms of quantities produced, 1,6-diaminohexane (a precursor to Nylon 6-6) is most important, followed by ethylenediamine. Vicinal diamines (1,2-diamines) are a structural motif in many biological compounds and are used as ligands in coordination chemistry. Aliphatic diamines Linear * 1 carbon: methylenediamine (diaminomethane) of theoretical interest only * 2 carbons: ethylenediamine (1,2-diaminoethane). Related derivatives include the N-alkylated compounds, 1,1-dimethylethylenediamine, 1,2-dimethylethylenediamine, ethambutol, tetrakis(dimethylamino)ethylene, TMEDA. File:Ethylene_diamine.png, Ethylenediamine * 3 carbons: 1,3-diaminopropane (propane-1,3-diamine) * 4 carbons: putrescine (butane-1,4-diamine) * 5 carbons: cadaverine (pentane-1,5-d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Production
Mass production, also known as flow production or continuous production, is the production of substantial amounts of standardized products in a constant flow, including and especially on assembly lines. Together with job production and batch production, it is one of the three main production methods. The term ''mass production'' was popularized by a 1926 article in the ''Encyclopædia Britannica'' supplement that was written based on correspondence with Ford Motor Company. ''The New York Times'' used the term in the title of an article that appeared before publication of the ''Britannica'' article. The concepts of mass production are applied to various kinds of products: from fluids and particulates handled in bulk ( food, fuel, chemicals and mined minerals), to parts and assemblies of parts (household appliances and automobiles). Some mass production techniques, such as standardized sizes and production lines, predate the Industrial Revolution by many centuries; howe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
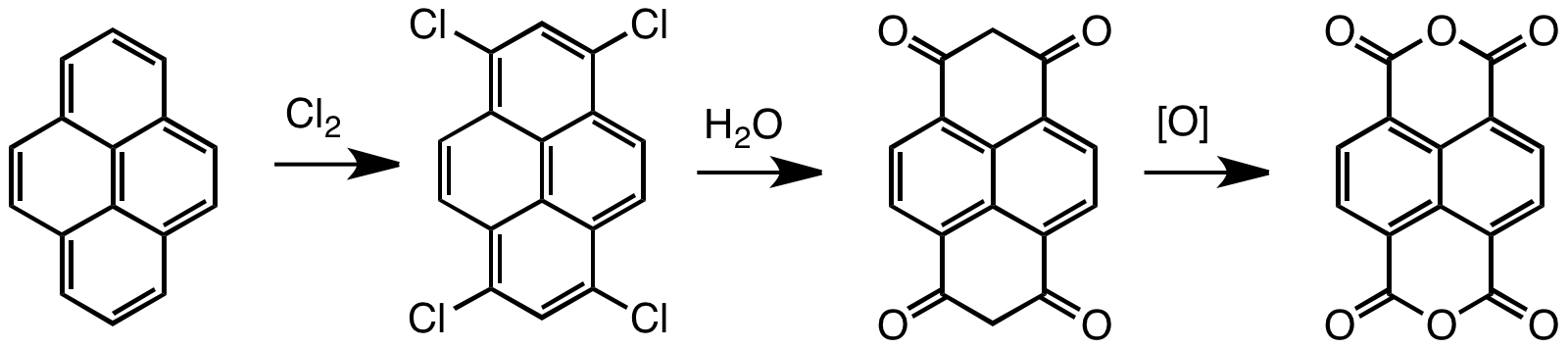
Naphthalene Tetracarboxylic Dianhydride
Naphthalenetetracarboxylic dianhydride (NTDA) is an organic compound related to naphthalene. The compound is a beige solid. NTDA is most commonly used as a precursor to naphthalenediimides (NDIs) (such as napthalenetetracarboxylic diimide), a family of compounds with many uses. Synthesis and structure Naphthalenetetracarboxylic dianhydride is prepared by oxidation of pyrene. Typical oxidants are chromic acid and chlorine. The unsaturated tetrachloride hydrolyzes to enols that tautomerize to the bis-dione, which in turn can be oxidized to the tetracarboxylic acid.F. Röhrscheid "Carboxylic Acids, Aromatic" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2012. Naphthalene diimides Symmetrical naphthalene diimides are synthesized by the condensation reaction of primary amines and the dianhydride. Unsymmetrical derivatives, i.e. those derived from two different amines, are obtained by hydrolysis of one of the two anhydride groups prior to the conde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Creep (deformation)
In materials science, creep (sometimes called cold flow) is the tendency of a solid material to move slowly or deform permanently under the influence of persistent mechanical stresses. It can occur as a result of long-term exposure to high levels of stress that are still below the yield strength of the material. Creep is more severe in materials that are subjected to heat for long periods and generally increases as they near their melting point. The rate of deformation is a function of the material's properties, exposure time, exposure temperature and the applied structural load. Depending on the magnitude of the applied stress and its duration, the deformation may become so large that a component can no longer perform its function – for example creep of a turbine blade could cause the blade to contact the casing, resulting in the failure of the blade. Creep is usually of concern to engineers and metallurgists when evaluating components that operate under high stresses or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_V1.png)