|
Plastic
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adaptability, plus a wide range of other properties, such as being lightweight, durable, flexible, and inexpensive to produce, has led to its widespread use. Plastics typically are made through human industrial systems. Most modern plastics are derived from fossil fuel-based chemicals like natural gas or petroleum; however, recent industrial methods use variants made from renewable materials, such as corn or cotton derivatives. 9.2 billion tonnes of plastic are estimated to have been made between 1950 and 2017. More than half this plastic has been produced since 2004. In 2020, 400 million tonnes of plastic were produced. If global trends on plastic demand continue, it is estimated that by 2050 annual global plastic production will reach ove ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plastic Pollution
Plastic pollution is the accumulation of plastic objects and particles (e.g. plastic bottles, bags and microbeads) in the Earth's environment that adversely affects humans, wildlife and their habitat. Plastics that act as pollutants are categorized by size into micro-, meso-, or macro debris. Plastics are inexpensive and durable, making them very adaptable for different uses; as a result, manufacturers choose to use plastic over other materials. However, the chemical structure of most plastics renders them resistant to many natural processes of degradation and as a result they are slow to degrade. Together, these two factors allow large volumes of plastic to enter the environment as mismanaged waste and for it to persist in the ecosystem. Plastic pollution can afflict land, waterways and oceans. It is estimated that 1.1 to 8.8 million tonnes of plastic waste enters the ocean from coastal communities each year. It is estimated that there is a stock of 86 million tons of plast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
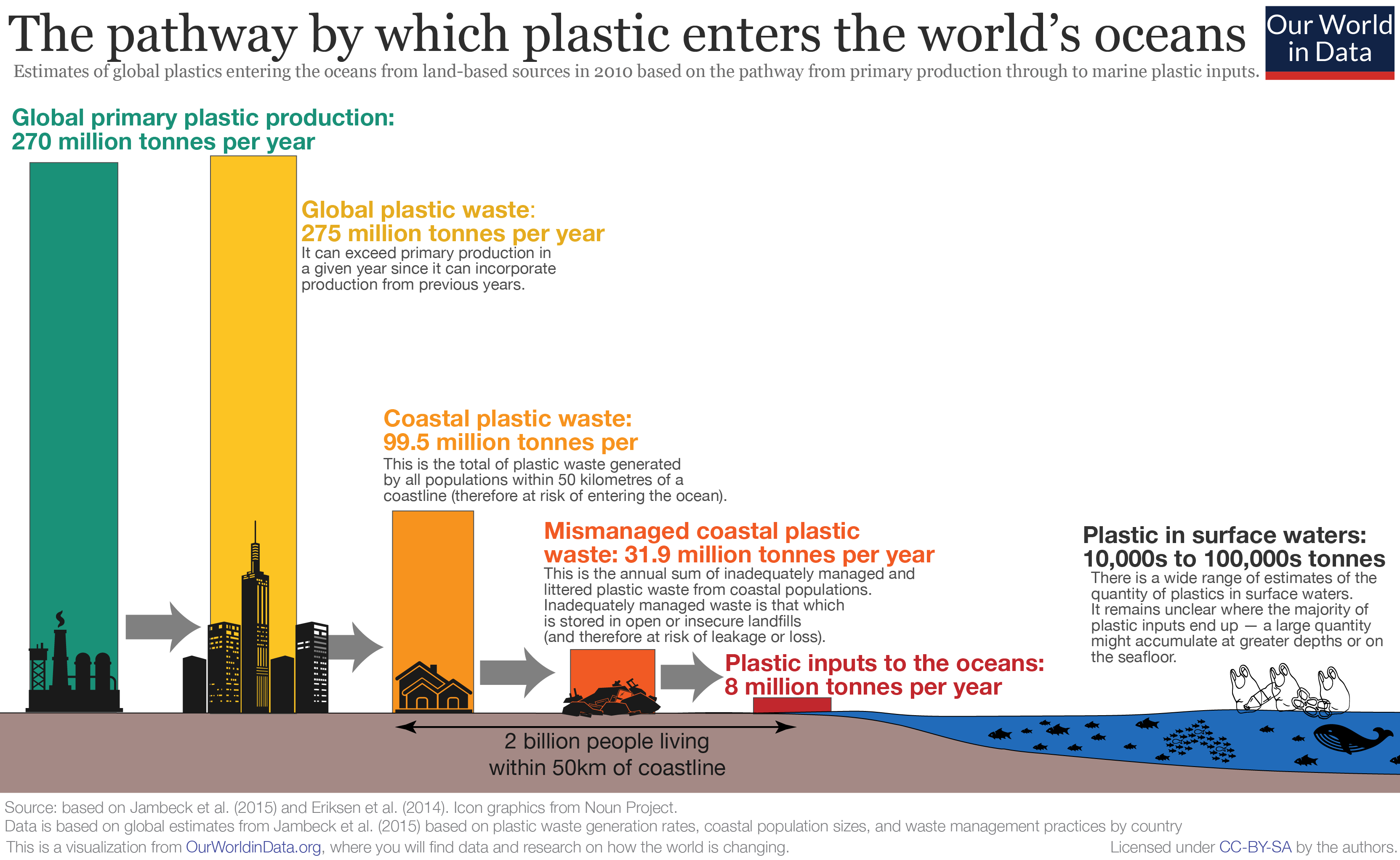
Marine Plastic Pollution
Marine plastic pollution (or plastic pollution in the ocean) is a type of marine pollution by plastics, ranging in size from large original material such as bottles and bags, down to microplastics formed from the fragmentation of plastic material. Marine debris is mainly discarded human rubbish which floats on, or is suspended in the ocean. Eighty percent of marine debris is plastic. Microplastics and nanoplastics result from the breakdown or photodegradation of plastic waste in surface waters, rivers or oceans. Recently, scientists have uncovered nanoplastics in heavy snow, more specifically about 3000 tons that cover Switzerland yearly. It is estimated that there is a stock of 86 million tons of plastic marine debris in the worldwide ocean as of the end of 2013, assuming that 1.4% of global plastics produced from 1950 to 2013 has entered the ocean and has accumulated there. It is estimated that 19–23 million tonnes of plastic leaks into aquatic ecosystems annually. The 2017 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plastic Household Items
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adaptability, plus a wide range of other properties, such as being lightweight, durable, flexible, and inexpensive to produce, has led to its widespread use. Plastics typically are made through human industrial systems. Most modern plastics are derived from fossil fuel-based chemicals like natural gas or petroleum; however, recent industrial methods use variants made from renewable materials, such as corn or cotton derivatives. 9.2 billion tonnes of plastic are estimated to have been made between 1950 and 2017. More than half this plastic has been produced since 2004. In 2020, 400 million tonnes of plastic were produced. If global trends on plastic demand continue, it is estimated that by 2050 annual global plastic production will reach over 1,1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plastic Recycling
Plastic recycling is the reprocessing of plastic waste into new products. When performed correctly, this can reduce dependence on landfill, conserve resources and protect the environment from plastic pollution and greenhouse gas emissions. Although recycling rates are increasing, they lag behind those of other recoverable materials, such as aluminium, glass and paper. Since the beginning of plastic production in the 20th century, until 2015, the world has produced some 6.3 billion tonnes of plastic waste, only 9% of which has been recycled, and only ~1% has been recycled more than once. Additionally, 12% was incinerated and the remaining 79% disposed of to landfill or to the environment including the sea. Recycling is necessary because almost all plastic is non-biodegradable and thus builds-up in the environment, where it can cause harm. For example, approximately 8 million tons of waste plastic enter the Earth's oceans every year, causing damage to the aquatic ecosystem and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including bottles, etc.). , over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market. Many kinds of polyethylene are known, with most having the chemical formula (C2H4)''n''. PE is usually a mixture of similar polymers of ethylene, with various values of ''n''. It can be ''low-density'' or ''high-density'': low-density polyethylene is extruded using high pressure () and high temperature (), while high-density polyethylene is extruded using low pressure () and low temperature (). Polyethylene is usually thermoplastic, but it can be modified to become thermosetting instead, for example, in cross-linked polyethylene. History Polyethylene was first synthesized by the German chemis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasticity (physics)
In physics and materials science, plasticity, also known as plastic deformation, is the ability of a solid material to undergo permanent deformation, a non-reversible change of shape in response to applied forces. For example, a solid piece of metal being bent or pounded into a new shape displays plasticity as permanent changes occur within the material itself. In engineering, the transition from elastic behavior to plastic behavior is known as yielding. Plastic deformation is observed in most materials, particularly metals, soils, rocks, concrete, and foams. However, the physical mechanisms that cause plastic deformation can vary widely. At a crystalline scale, plasticity in metals is usually a consequence of dislocations. Such defects are relatively rare in most crystalline materials, but are numerous in some and part of their crystal structure; in such cases, plastic crystallinity can result. In brittle materials such as rock, concrete and bone, plasticity is caused ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Garbage Patch
A garbage patch is a gyre of marine debris particles caused by the effects of ocean currents and increasing plastic pollution by human populations. These human-caused collections of plastic and other debris, cause ecosystem and environmental problems that affect marine life, contaminate oceans with toxic chemicals, and contribute to greenhouse gas emissions. Once waterborne, marine debris becomes mobile. Flotsam can be blown by the wind, or follow the flow of ocean currents, often ending up in the middle of oceanic gyres where currents are weakest. Garbage patches grow because of widespread loss of plastic from human trash collection systems. The United Nations Environmental Program estimated that "for every square mile of ocean" there are about "46,000 pieces of plastic." The 10 largest emitters of oceanic plastic pollution worldwide are, from the most to the least, China, Indonesia, Philippines, Vietnam, Sri Lanka, Thailand, Egypt, Malaysia, Nigeria, and Bangladesh, largely thr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Injection Moulding
Injection moulding (U.S. spelling: injection molding) is a manufacturing process for producing parts by injecting molten material into a mould, or mold. Injection moulding can be performed with a host of materials mainly including metals (for which the process is called die-casting), glasses, elastomers, confections, and most commonly thermoplastic and thermosetting polymers. Material for the part is fed into a heated barrel, mixed (using a helical screw), and injected into a mould cavity, where it cools and hardens to the configuration of the cavity. After a product is designed, usually by an industrial designer or an engineer, moulds are made by a mould-maker (or toolmaker) from metal, usually either steel or aluminium, and precision-machined to form the features of the desired part. Injection moulding is widely used for manufacturing a variety of parts, from the smallest components to entire body panels of cars. Advances in 3D printing technology, using photopolymers t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plastics Industry
The plastics industry manufactures polymer materials—commonly called plastics—and offers services in plastics important to a range of industries, including packaging, building and construction, electronics, aerospace, and transportation. It is part of the chemical industry. In addition, as mineral oil is the major constituent of plastics, it therefore forms part of the petrochemical industry. Besides plastics production, plastics engineering is an important part of the industrial sector. The latter field is dominated by engineering plastic as raw material because of its better mechanical and thermal properties than the more widely used commodity plastics. Companies Markets According to PlasticsEurope, the top three markets for plastics are packaging, building and construction, and automotive. Production Plastics production has been growing globally. The numbers include thermoplastics and polyurethanes, as well as thermosets, adhesives, coatings and sealants and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bakelite
Polyoxybenzylmethylenglycolanhydride, better known as Bakelite ( ), is a thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde. The first plastic made from synthetic components, it was developed by Leo Baekeland in Yonkers, New York in 1907, and patented on December 7, 1909 (). Because of its electrical nonconductivity and heat-resistant properties, it became a great commercial success. It was used in electrical insulators, radio and telephone casings, and such diverse products as kitchenware, jewelry, pipe stems, children's toys, and firearms. The "retro" appeal of old Bakelite products has made them collectible. The creation of a synthetic plastic was revolutionary for the chemical industry, which at the time made most of its income from cloth dyes and explosives. Bakelite's commercial success inspired the industry to develop other synthetic plastics. In recognition of its significance as the world's first commercial syntheti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plumbing
Plumbing is any system that conveys fluids for a wide range of applications. Plumbing uses pipes, valves, plumbing fixtures, tanks, and other apparatuses to convey fluids. Heating and cooling (HVAC), waste removal, and potable water delivery are among the most common uses for plumbing, but it is not limited to these applications. The word derives from the Latin for lead, ''plumbum'', as the first effective pipes used in the Roman era were lead pipes. In the developed world, plumbing infrastructure is critical to public health and sanitation. Boilermakers and pipefitters are not plumbers although they work with piping as part of their trade and their work can include some plumbing. History Plumbing originated during ancient civilizations, as they developed public baths and needed to provide potable water and wastewater removal for larger numbers of people. The Mesopotamians introduced the world to clay sewer pipes around 4000 BCE, with the earliest exampl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |