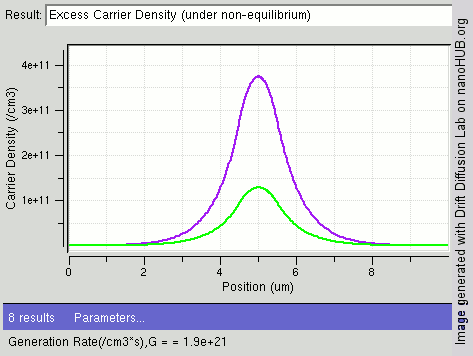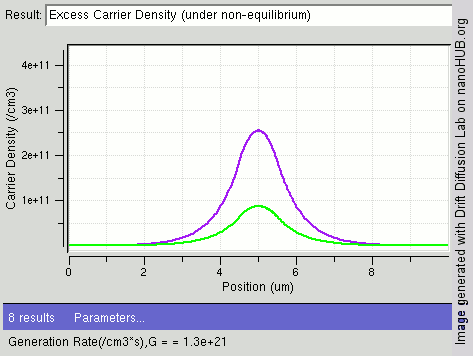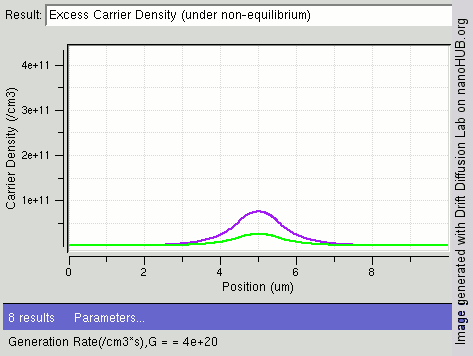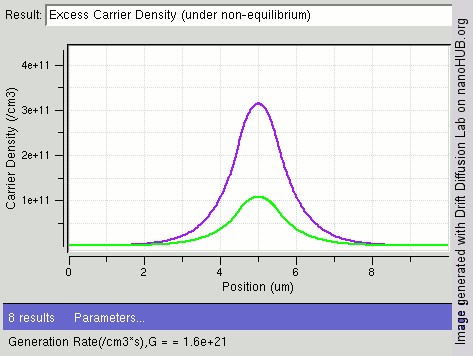
|
Perovskite Solar Cell
A perovskite solar cell (PSC) is a type of solar cell that includes a perovskite-structured compound, most commonly a hybrid organic–inorganic lead or tin halide-based material as the light-harvesting active layer. Perovskite materials, such as methylammonium lead halides and all-inorganic caesium lead halide, are cheap to produce and simple to manufacture. Efficiencies of laboratory-scale devices using these materials have increased from 3.8% in 2009 to 25.7% in 2021 in single-junction architectures, and, in silicon-based tandem cells, to 29.8%, exceeding the maximum efficiency achieved in single-junction silicon solar cells. Perovskite solar cells have therefore been the fastest-advancing solar technology . With the potential of achieving even higher efficiencies and very low production costs, perovskite solar cells have become commercially attractive. Core problems and research subjects include their short- and long-term stability. Advantages The raw materials used and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perovskite Solar Cells 1
Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula ). Its name is also applied to the class of compounds which have the same type of crystal structure as (XIIA2+VIB4+X2−3), known as the perovskite structure. Many different cations can be embedded in this structure, allowing the development of diverse engineered materials. History The mineral was discovered in the Ural Mountains of Russia by Gustav Rose in 1839 and is named after Russian mineralogist Lev Perovski (1792–1856). Perovskite's notable crystal structure was first described by Victor Goldschmidt in 1926 in his work on tolerance factors. The crystal structure was later published in 1945 from X-ray diffraction data on barium titanate by Helen Dick Megaw. Occurrence Found in the Earth's mantle, perovskite's occurrence at Khibina Massif is restricted to the silica under-saturated ultramafic rocks and foidolites, due to the instability in a paragenesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the concept of space groups. All possible symmetric arrangements of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Multi-junction Solar Cell
Multi-junction (MJ) solar cells are solar cells with multiple p–n junctions made of different semiconductor materials. Each material's p-n junction will produce electric current in response to different wavelengths of light. The use of multiple semiconducting materials allows the absorbance of a broader range of wavelengths, improving the cell's sunlight to electrical energy conversion efficiency. Traditional single-junction cells have a maximum theoretical efficiency of 33.16%. Theoretically, an infinite number of junctions would have a limiting efficiency of 86.8% under highly concentrated sunlight. As of 2008 the best lab examples of traditional crystalline silicon (c-Si) solar cells had efficiencies between 20% and 25%, while lab examples of multi-junction cells have demonstrated performance over 46% under concentrated sunlight. Commercial examples of tandem cells are widely available at 30% under one-sun illumination, and improve to around 40% under concentrated sunlight. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiative Recombination
In the solid-state physics of semiconductors, carrier generation and carrier recombination are processes by which mobile charge carriers (electrons and electron holes) are created and eliminated. Carrier generation and recombination processes are fundamental to the operation of many optoelectronic semiconductor devices, such as photodiodes, light-emitting diodes and laser diodes. They are also critical to a full analysis of p-n junction devices such as bipolar junction transistors and p-n junction diodes. The electron–hole pair is the fundamental unit of generation and recombination in inorganic semiconductors, corresponding to an electron transitioning between the valence band and the conduction band where generation of electron is a transition from the valence band to the conduction band and recombination leads to a reverse transition. Overview Like other solids, semiconductor materials have an electronic band structure determined by the crystal properties of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P–n Junction
A p–n junction is a boundary or interface between two types of semiconductor materials, p-type and n-type, inside a single crystal of semiconductor. The "p" (positive) side contains an excess of holes, while the "n" (negative) side contains an excess of electrons in the outer shells of the electrically neutral atoms there. This allows electrical current to pass through the junction only in one direction. The p-n junction is created by doping, for example by ion implantation, diffusion of dopants, or by epitaxy (growing a layer of crystal doped with one type of dopant on top of a layer of crystal doped with another type of dopant). If two separate pieces of material were used, this would introduce a grain boundary between the semiconductors that would severely inhibit its utility by scattering the electrons and holes. p–n junctions are elementary "building blocks" of semiconductor electronic devices such as diodes, transistors, solar cells, light-emitting diodes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shockley–Queisser Limit
In physics, the radiative efficiency limit (also known as the detailed balance limit, Shockley–Queisser limit, Shockley Queisser Efficiency Limit or SQ Limit) is the maximum theoretical efficiency of a solar cell using a single p-n junction to collect power from the cell where the only loss mechanism is radiative recombination in the solar cell. It was first calculated by William Shockley and Hans-Joachim Queisser at Shockley Semiconductor in 1961, giving a maximum efficiency of 30% at 1.1 eV. The limit is one of the most fundamental to solar energy production with photovoltaic cells, and is considered to be one of the most important contributions in the field. This first calculation used the 6000K black-body spectrum as an approximation to the solar spectrum. Subsequent calculations have used measured global solar spectra, AM 1.5, and included a back surface mirror which increases the maximum solar conversion efficiency to 33.16% for a single-junction solar cell with a b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Single-junction Cell
A unijunction transistor (UJT) is a three-lead electronic semiconductor device with only one junction that acts exclusively as an electrically controlled switch. The UJT is not used as a linear amplifier. It is used in free-running oscillators, synchronized or triggered oscillators, and pulse generation circuits at low to moderate frequencies (hundreds of kilohertz). It is widely used in the triggering circuits for silicon controlled rectifiers. In the 1960s, the low cost per unit, combined with its unique characteristic, warranted its use in a wide variety of applications like oscillators, pulse generators, saw-tooth generators, triggering circuits, phase control, timing circuits, and voltage- or current-regulated supplies.J. F. Cleary (ed.), ''General Electric Transistor Manual'', General Electric, 1964 Chapter 13 "Unijunction Transistor Circuits" The original unijunction transistor types are now considered obsolete, but a later multi-layer device, the programmable unijunction tra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bandgap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in electron volts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote a valence electron bound to an atom to become a conduction electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there is no generated current due to no net charge carrier mobility. However, if some electrons transfer fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant materials, and cell stains. Although uncommon, chronic toxicity from bromide can result in bromism, a syndrome with multiple neurological symptoms. Bromide toxicity can also cause a type of skin eruption, see potassium bromide. The bromide ion has an ionic radius of 196 pm. Natural occurrence Bromide is present in typical seawater (35 PSU) with a concentration of around 65 mg/L, which is about 0.2% of all dissolved salts. Seafood and deep sea plants generally have higher levels than land-derived foods. Bromargyrite—natural, crystalline silver bromide—is the most common bromide mineral known but is still very rare. In addition to silver, bromine is also in minerals combined with mercury and copper. Formation and re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_crystallographic_standard_alignment.png)