|
Nanomaterials
* Nanomaterials describe, in principle, materials of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of nanoscale). Nanomaterials research takes a materials science-based approach to nanotechnology, leveraging advances in materials metrology and synthesis which have been developed in support of microfabrication research. Materials with structure at the nanoscale often have unique optical, electronic, thermo-physical or mechanical properties. Nanomaterials are slowly becoming commercialized and beginning to emerge as commodities. Definition In ISO/TS 80004, ''nanomaterial'' is defined as the "material with any external dimension in the nanoscale or having internal structure or surface structure in the nanoscale", with ''nanoscale'' defined as the "length range approximately from 1 nm to 100 nm". This includes both ''nano-objects'', which are discrete pieces of material, and ''nanostructured materials'', which have i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanotechnology
Nanotechnology, also shortened to nanotech, is the use of matter on an atomic, molecular, and supramolecular scale for industrial purposes. The earliest, widespread description of nanotechnology referred to the particular technological goal of precisely manipulating atoms and molecules for fabrication of macroscale products, also now referred to as molecular nanotechnology. A more generalized description of nanotechnology was subsequently established by the National Nanotechnology Initiative, which defined nanotechnology as the manipulation of matter with at least one dimension sized from 1 to 100 nanometers (nm). This definition reflects the fact that quantum mechanical effects are important at this quantum-realm scale, and so the definition shifted from a particular technological goal to a research category inclusive of all types of research and technologies that deal with the special properties of matter which occur below the given size threshold. It is therefore commo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Dioxide Nanoparticle
Titanium dioxide nanoparticles, also called ultrafine titanium dioxide or nanocrystalline titanium dioxide or microcrystalline titanium dioxide, are particles of titanium dioxide () with diameters less than 100 nm. Ultrafine is used in sunscreens due to its ability to block ultraviolet radiation while remaining transparent on the skin. It is in rutile crystal structure and coated with silica or/and alumina to prevent photocatalytic phenomena. The health risks of ultrafine from dermal exposure on intact skin are considered extremely low, and it is considered safer than other substances used for ultraviolet protection. Nanosized particles of titanium dioxide tend to form in the metastable anatase phase, due to the lower surface energy of this phase, relative to the equilibrium rutile phase. Surfaces of ultrafine titanium dioxide in the anatase structure have photocatalytic sterilizing properties, which make it useful as an additive in construction materials, for example in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultrafine Particle
Ultrafine particles (UFPs) are particulate matter of nanoscale size (less than 0.1 μm or 100 nm in diameter). Regulations do not exist for this size class of ambient air pollution particles, which are far smaller than the regulated PM10 and PM2.5 particle classes and are believed to have several more aggressive health implications than those classes of larger particulates. In the EU UFP's in ambient air are empirically defined by technical specification The important detail is the definition of size, stated: "The lower and upper sizes considered within this document are 7 nm and a few micrometres, respectively". Although the most common referral to UFP is "less than 0.1μm", this is incorrect for ambient air in the EU. There are two main divisions that categorize types of UFPs. UFPs can either be carbon-based or metallic, and then can be further subdivided by their magnetic properties. Electron microscopy and special physical lab conditions allow scientists to observe UFP morp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
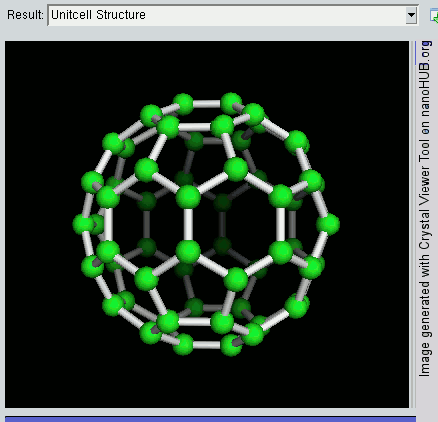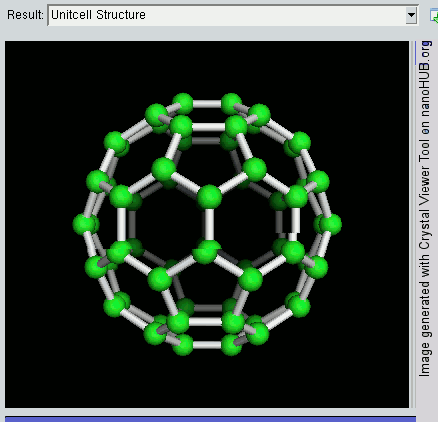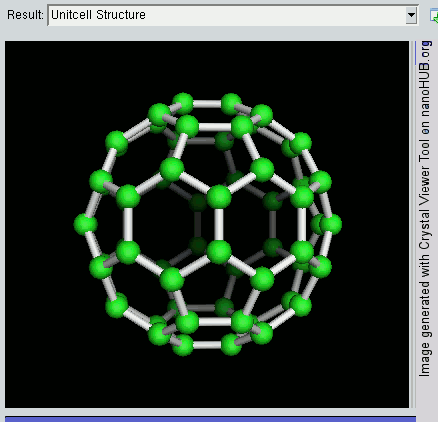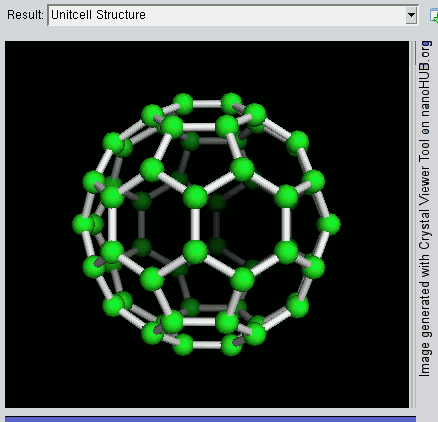
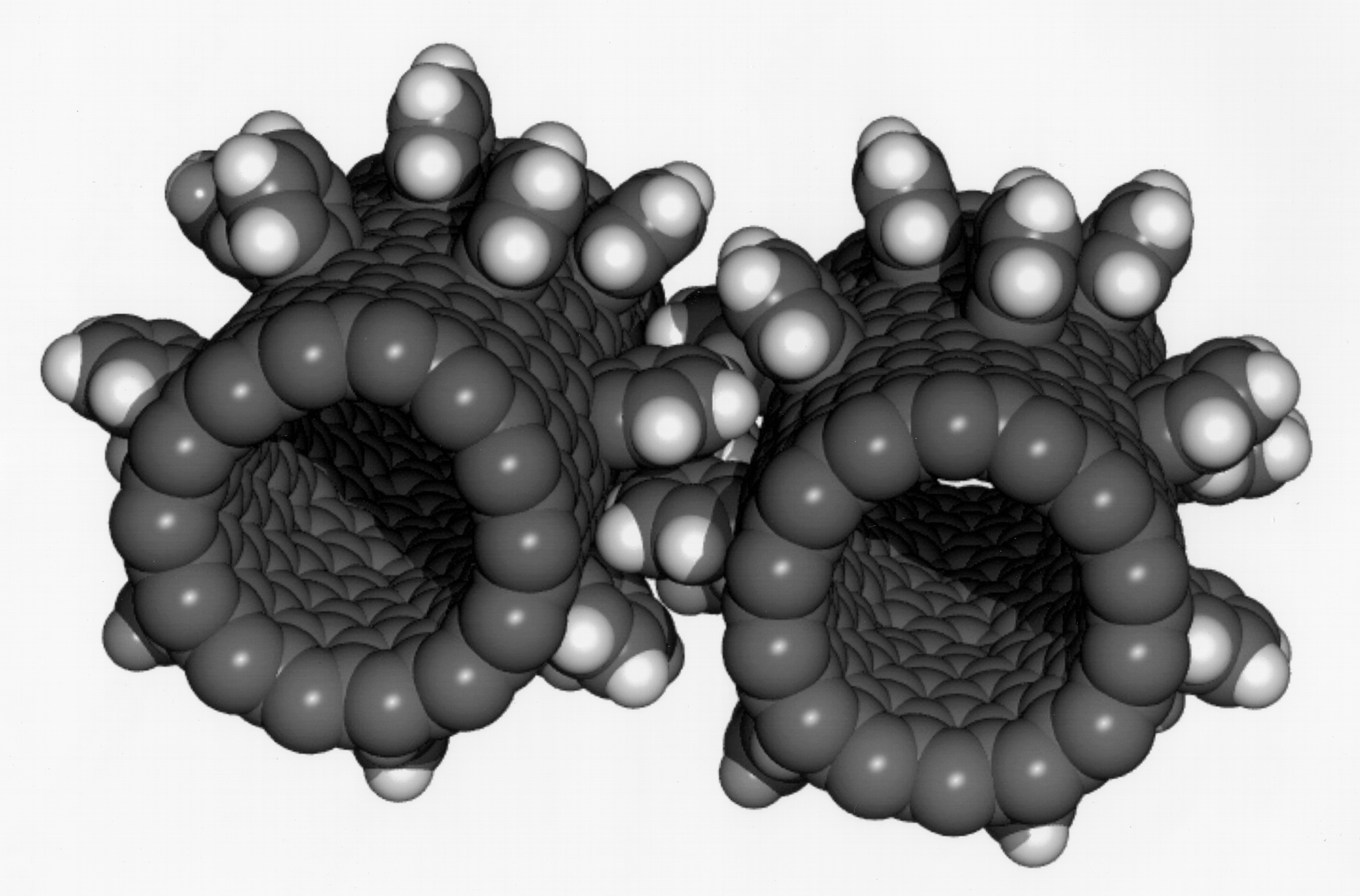
Fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ellipsoid, tube, or many other shapes and sizes. Graphene (isolated atomic layers of graphite), which is a flat mesh of regular hexagonal rings, can be seen as an extreme member of the family. Fullerenes with a closed mesh topology are informally denoted by their empirical formula C''n'', often written C''n'', where ''n'' is the number of carbon atoms. However, for some values of ''n'' there may be more than one isomer. The family is named after buckminsterfullerene (C60), the most famous member, which in turn is named after Buckminster Fuller. The closed fullerenes, especially C60, are also informally called buckyballs for their resemblance to the standard ball of association football ("soccer"). Nested closed fullerenes have been na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gecko
Geckos are small, mostly carnivorous lizards that have a wide distribution, found on every continent except Antarctica. Belonging to the infraorder Gekkota, geckos are found in warm climates throughout the world. They range from . Geckos are unique among lizards for their vocalisations, which differ from species to species. Most geckos in the family Gekkonidae use chirping or clicking sounds in their social interactions. Tokay geckos (''Gekko gecko'') are known for their loud mating calls, and some other species are capable of making hissing noises when alarmed or threatened. They are the most species-rich group of lizards, with about 1,500 different species worldwide. All geckos, except species in the family Eublepharidae lack eyelids; instead, the outer surface of the eyeball has a transparent membrane, the cornea. They have a fixed lens within each iris that enlarges in darkness to let in more light. Since they cannot blink, species without eyelids generally ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capsid
A capsid is the protein shell of a virus, enclosing its genetic material. It consists of several oligomeric (repeating) structural subunits made of protein called protomers. The observable 3-dimensional morphological subunits, which may or may not correspond to individual proteins, are called capsomeres. The proteins making up the capsid are called capsid proteins or viral coat proteins (VCP). The capsid and inner genome is called the nucleocapsid. Capsids are broadly classified according to their structure. The majority of the viruses have capsids with either helical or icosahedral structure. Some viruses, such as bacteriophages, have developed more complicated structures due to constraints of elasticity and electrostatics. The icosahedral shape, which has 20 equilateral triangular faces, approximates a sphere, while the helical shape resembles the shape of a spring, taking the space of a cylinder but not being a cylinder itself. The capsid faces may consist of one or m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nelumbo Nucifera
''Nelumbo nucifera'', also known as sacred lotus, Laxmi lotus, Indian lotus, or simply lotus, is one of two extant species of aquatic plant in the family Nelumbonaceae. It is sometimes colloquially called a water lily, though this more often refers to members of the family Nymphaeaceae. Lotus plants are adapted to grow in the flood plains of slow-moving rivers and delta areas. Stands of lotus drop hundreds of thousands of seeds every year to the bottom of the pond. While some sprout immediately, and most are eaten by wildlife, the remaining seeds can remain dormant for an extensive period of time as the pond silts in and dries out. During flood conditions, sediments containing these seeds are broken open, and the dormant seeds rehydrate and begin a new lotus colony. Under favorable circumstances, the seeds of this aquatic perennial may remain viable for many years, with the oldest recorded lotus germination being from seeds 1,300 years old recovered from a dry lakebed i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropaeolum
''Tropaeolum'' , commonly known as nasturtium (; literally "nose-twister" or "nose-tweaker"), is a genus of roughly 80 species of annual and perennial herbaceous flowering plants. It was named by Carl Linnaeus in his book ''Species Plantarum'', and is the only genus in the family Tropaeolaceae. The nasturtiums received their common name because they produce an oil similar to that of watercress (''Nasturtium officinale''). The genus ''Tropaeolum'', native to South and Central America, includes several very popular garden plants, the most common being '' T. majus'', '' T. peregrinum'' and '' T. speciosum''. One of the hardiest species is '' T. polyphyllum'' from Chile, the perennial roots of which can survive the winter underground at elevations of . Plants in this genus have showy, often intensely bright flowers and rounded, peltate (shield-shaped) leaves with the petiole in the centre. The flowers are bisexual and zygomorphic, with five petals, a superior three-carpelled ov ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Milk
Milk is a white liquid food produced by the mammary glands of mammals. It is the primary source of nutrition for young mammals (including breastfed human infants) before they are able to digest solid food. Immune factors and immune-modulating components in milk contribute to milk immunity. Early- lactation milk, which is called colostrum, contains antibodies that strengthen the immune system, and thus reduces the risk of many diseases. Milk contains many nutrients, including protein and lactose. As an agricultural product, dairy milk is collected from farm animals. In 2011, dairy farms produced around of milk from 260 million dairy cows. India is the world's largest producer of milk and the leading exporter of skimmed milk powder, but it exports few other milk products. Because there is an ever-increasing demand for dairy products within India, it could eventually become a net importer of dairy products. New Zealand, Germany and the Netherlands are the largest expo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morpho Rhetenor
''Morpho rhetenor'', the Rhetenor blue morpho, is a Neotropical butterfly of the family Nymphalidae. It is found in Suriname, French Guiana, Brazil, Peru, Ecuador, Colombia, and Venezuela. Description Hans Fruhstorfer describes: "''M. rhetenor'', already named by Cramer the 'blue elongate Atlas butterfly', has the apex of the forewing more produced than any other Morphid species; a characteristic, however, that partially disappears in the female, which more resembles that of '' cypris''. The male is one of the most brilliantly glossy species and has only a quite inconsiderable black apical spot and a white costal patch on the forewing. The under surface is noteworthy for the contrast between the black basal area and a brown distal region, which are separated by a median band of a more or less pure white and of varying extent according to the locality. Both wings beneath show brown rounded eye-spots entirely without white pupils." Sex difference ''Morpho rhetenor'' is sexually di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Council On Radiation Protection And Measurements
The National Council on Radiation Protection and Measurements (NCRP), formerly the National Committee on Radiation Protection and Measurements, and before that the Advisory Committee on X-Ray and Radium Protection (ACXRP), is a U.S. organization. It has a congressional charter under Title 36 of the United States Code The United States Code is the official compilation of the Federal laws of a general and permanent nature that are currently in force. Title 36 cover, "Patriotic and National Observances, Ceremonies, and Organizations." Parts Subtitle I: Patrio ..., but this does not imply any sort of oversight by Congress; NCRP is not a government entity. History The Advisory Committee on X-Ray and Radium Protection was established in 1929. Initially, the organization was an informal collective of scientists seeking to proffer accurate information and appropriate recommendations for radiation protection. In 1946, the organization changed its name to the National Committee on Radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the circulatory system is also known as ''peripheral blood'', and the blood cells it carries, ''peripheral blood cells''. Blood is composed of blood cells suspended in blood plasma. Plasma, which constitutes 55% of blood fluid, is mostly water (92% by volume), and contains proteins, glucose, mineral ions, hormones, carbon dioxide (plasma being the main medium for excretory product transportation), and blood cells themselves. Albumin is the main protein in plasma, and it functions to regulate the colloidal osmotic pressure of blood. The blood cells are mainly red blood cells (also called RBCs or erythrocytes), white blood cells (also called WBCs or leukocytes) and platelets (also called thrombocytes). The most abundant cells in vertebrate blood ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.gif)


.jpg)

