|
McCabe–Thiele Method
The McCabe–Thiele method is a chemical engineering technique for the analysis of binary distillation. It uses the fact that the composition at each theoretical tray (or equilibrium stage) is completely determined by the mole fraction of one of the two components and is based on the assumption of constant molar overflow, which requires that: * the molar heats of vaporization of the feed components are equal; * for every mole of liquid vaporized, a mole of vapor is condensed; and * heat effects such as heat of solution are negligible. The method was first published by Warren L. McCabe and Ernest Thiele in 1925, both of whom were working at the Massachusetts Institute of Technology (MIT) at the time. Construction and use A McCabe–Thiele diagram for the distillation of a binary feed is constructed using the vapor-liquid equilibrium (VLE) data for the lower-boiling component of the feed. On a planar graph, the horizontal (x) axis represents the mole fraction of the liqu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Engineering
Chemical engineering is an engineering field which deals with the study of operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials into useful products. Chemical engineering uses principles of chemistry, physics, mathematics, biology, and economics to efficiently use, produce, design, transport and transform energy and materials. The work of chemical engineers can range from the utilization of nanotechnology and nanomaterials in the laboratory to large-scale industrial processes that convert chemicals, raw materials, living cells, microorganisms, and energy into useful forms and products. Chemical engineers are involved in many aspects of plant design and operation, including safety and hazard assessments, process design and analysis, modeling, control engineering, chemical reaction engineering, nuclear engineering, biological engineering, construction specificatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Massachusetts Institute Of Technology
The Massachusetts Institute of Technology (MIT) is a private land-grant research university in Cambridge, Massachusetts. Established in 1861, MIT has played a key role in the development of modern technology and science, and is one of the most prestigious and highly ranked academic institutions in the world. Founded in response to the increasing industrialization of the United States, MIT adopted a European polytechnic university model and stressed laboratory instruction in applied science and engineering. MIT is one of three private land grant universities in the United States, the others being Cornell University and Tuskegee University. The institute has an urban campus that extends more than a mile (1.6 km) alongside the Charles River, and encompasses a number of major off-campus facilities such as the MIT Lincoln Laboratory, the Bates Center, and the Haystack Observatory, as well as affiliated laboratories such as the Broad and Whitehead Institutes. , 98 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fractional Distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used. It is used to refine crude oil. Laboratory setup Fractional distillation in a laboratory makes use of common laboratory glassware and apparatuses, typically including a Bunsen burner, a round-bottomed flask and a condenser, as well as the single-purpose fractionating column. As an example, consider the distillation of a mixture of water and ethanol. Ethanol boils at while water boils at . So, by heating the mixture, the most volatile component (ethanol) will ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
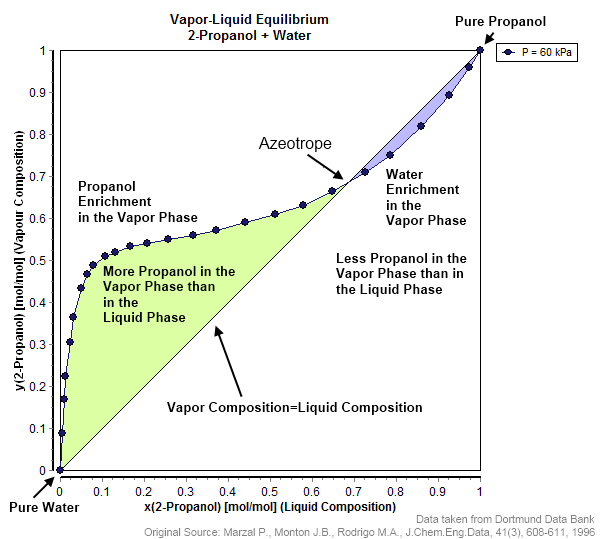
Azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Because their composition is unchanged by distillation, azeotropes are also called (especially in older texts) ''constant boiling point mixtures''. Some azeotropic mixtures of pairs of compounds are known, and many azeotropes of three or more compounds are also known. In such a case it is not possible to separate the components by fractional distillation and azeotropic distillation is usually used instead. There are two types of azeotropes: minimum boiling azeotrope and maximum boiling azeotrope. A solution that shows greater positive deviation from Raoult's law forms a minimum boiling azeotrope at a specific composition. For ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Continuous Distillation
Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously (without interruption) fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling (or evaporation) and condensation. The process produces at least two output fractions. These fractions include at least one '' volatile'' distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a ''bottoms'' (or ''residuum'') fraction, which is the least volatile residue that has not been separately captured as a condensed vapor. An alternative to continuous distillation is batch distillation, where the mixture is added to the unit at the start of the distillation, distillate fractions are taken out sequentially in time (one after another) during the distillation, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Q-line Slopes
An IRCd, short for Internet Relay Chat daemon, is server software that implements the IRC protocol, enabling people to talk to each other via the Internet (exchanging textual messages in real time). It is distinct from an IRC bot that connects outbound to an IRC channel. The server listens to connections from IRC clients on a set of TCP ports. When the server is part of an IRC network, it also keeps one or more established connections to other servers/daemons. The term ''ircd'' originally referred to only one single piece of software, but it eventually became a generic reference to any implementation of an IRC daemon. However, the original version is still distributed under the same name, and this article discusses both uses. History The original IRCd was known as 'ircd', and was authored by Jarkko Oikarinen (WiZ on IRC) in 1988. He received help from a number of others, such as Markku Savela (msa on IRC), who helped with the 2.2+msa release, etc. In its first revisions, IR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time. Reflux in industrial distillation The term ''reflux'' is very widely used in industries that utilize large-scale distillation columns and fractionators such as petroleum refineries, petrochemical and chemical plants, and natural gas processing plants. In that context, reflux refers to the portion of the overhead liquid product from a distillation column or fractionator that is returned to the upper part of the column as shown in the schematic diagram of a typical industrial distillation column. Inside the column, the downflowing reflux liquid provides cooling and condensation of the upflowing vapors thereby increasing the efficiency of the distillation column. The more reflux provided for a given numb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation Column
A fractionating column or fractional column is an essential item used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on the differences in volatilities. Fractionating columns are used in small scale laboratory distillations as well as large scale industrial distillations. Laboratory fractionating columns A laboratory fractionating column is a piece of glassware used to separate vaporized mixtures of liquid compounds with close volatility. Most commonly used is either a Vigreux column or a straight column packed with glass beads or metal pieces such as Raschig rings. Fractionating columns help to separate the mixture by allowing the mixed vapors to cool, condense, and vaporize again in accordance with Raoult's law. With each condensation-vaporization cycle, the vapors are enriched in a certain component. A larger surface area allows more cycles, improving separation. This is the rationale for a Vigreux column or a pack ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor–liquid Equilibrium
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase. The concentration of a vapor in contact with its liquid, especially at equilibrium, is often expressed in terms of vapor pressure, which will be a partial pressure (a part of the total gas pressure) if any other gas(es) are present with the vapor. The equilibrium vapor pressure of a liquid is in general strongly dependent on temperature. At vapor–liquid equilibrium, a liquid with individual components in certain concentrations will have an equilibrium vapor in which the concentrations or partial pressures of the vapor components have certain values depending on all of the liquid component concentrations and the temperature. The converse is also true: if a vapor with components at certain concentrations or partial pressures is in vapor–liquid equilibrium with its liquid, then the component concentrati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ernest Thiele
Ernest W. Thiele (pronounced ; 1895–1993) was an influential chemical engineering researcher at Standard Oil (then Amoco, now BP) and Professor of Chemical Engineering at the University of Notre Dame. He is known for his highly impactful work in chemical reaction engineering, complex reacting systems, and separations, including distillation theory. Early life and education Ernest Thiele, born on December 8, 1895, grew up in Chicago, Illinois. In 1916, he earned an A.B. degree from Loyola University in Chicago. He was stationed at the University of Illinois at Urbana–Champaign with the U.S. Army, where he completed a bachelor's degree in chemical engineering in 1919. In the fall of 1922, matriculated to MIT where he began graduate studies under the direction of Professor Robert T. Haslam; he earned his M.S. degree in 1923 and a doctoral degree in 1925 with a thesis on steam-carbon reactions. Career Before starting his graduate degree at MIT, Thiele worked for six months fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids); this may involve chemical changes such as destructive distillation or cracking. Distillation may result in essentially complete separation (resulting in nearly pure components), or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction. An installation used for distillation, especially of distilled beverages, is a distillery. Distillation includes th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)