|
Hydrotrope
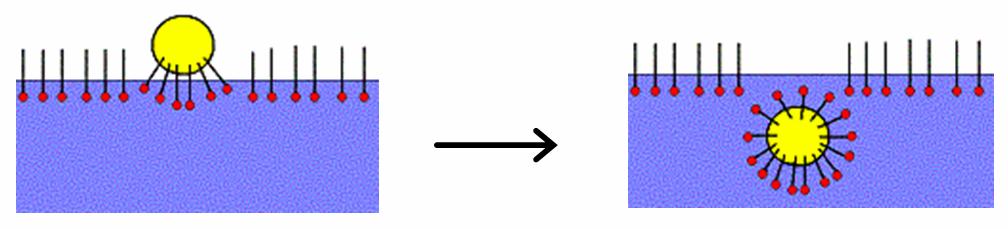
A hydrotrope is a compound that solubilizes hydrophobic compounds in aqueous solutions by means other than micellar solubilization. Typically, hydrotropes consist of a hydrophilic part and a hydrophobic part (similar to surfactants), but the hydrophobic part is generally too small to cause spontaneous self-aggregation. Hydrotropes do not have a critical concentration above which self-aggregation spontaneously starts to occur (as found for micelle- and vesicle-forming surfactants, which have a critical micelle concentration (cmc) and a critical vesicle concentration (cvc)). Instead, some hydrotropes aggregate in a step-wise self-aggregation process, gradually increasing aggregation size. However, many hydrotropes do not seem to self-aggregate at all, unless a solubilizate has been added. Examples of hydrotropes include urea, tosylate, cumenesulfonate and xylenesulfonate. The term ''hydrotropy'' was originally put forward by Carl Neuberg to describe the increase in the solubility o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Micellar Solubilization
Micellar solubilization (solubilization) is the process of incorporating the solubilizate (the component that undergoes solublization) into or onto micelles. Solublization may occur in a system consisting of a solvent, an association colloid (a colloid that forms micelles), and at least one other solubilizate. Usage of the term Solubilization is distinct from dissolution because the resulting fluid is a colloidal dispersion involving an association colloid. This suspension is distinct from a true solution, and the amount of the solubilizate in the micellar system can be different (often higher) than the regular solubility of the solubilizate in the solvent. In non-chemical literature and in everyday language, the term "solubilization" is sometimes used in a broader meaning as "to bring to a solution or (non- sedimenting) suspension" by any means, e.g., leaching by a reaction with an acid. Application Micellar solubilization is widely utilized, e.g. in laundry washing using ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactants
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants. The word "surfactant" is a blend of ''surface-active agent'', coined . Agents that increase surface tension are "surface active" in the literal sense but are not called surfactants as their effect is opposite to the common meaning. A common example of surface tension increase is salting out: by adding an inorganic salt to an aqueous solution of a weakly polar substance, the substance will precipitate. The substance may itself be a surfactant – this is one of the reasons why many surfactants are ineffective in sea water. Composition and structure Surfactants are usually organic compounds that are amphiphilic, meaning each molecule contains both a hydrophilic "water-seeking" group (the ''head''), and a hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Benzoate
Sodium benzoate is the sodium salt of benzoic acid, widely used as a food preservative (with an E number of E211) and a pickling agent. It appears as a white crystalline chemical with the formula C6H5COONa. Production Sodium benzoate is commonly produced by the neutralization of sodium hydroxide (NaOH) with benzoic acid (C6H5COOH), which is itself produced commercially by partial oxidation of toluene with oxygen. Natural occurrence Many foods are natural sources of benzoic acid, its salts, and its esters. Fruits and vegetables can be rich sources, particularly berries such as cranberry and bilberry. Other sources include seafood, such as prawns, and dairy products. Uses As a preservative Sodium benzoate can act as a food preservative. It is most widely used in acidic foods such as salad dressings (for example acetic acid in vinegar), carbonated drinks ( carbonic acid), jams and fruit juices (citric acid), pickles (acetic acid), condiments, and frozen yogurt toppings. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octanol-water Partition Coefficient
The ''n''-octanol-water partition coefficient, ''K''ow is a partition coefficient for the two-phase system consisting of ''n''-octanol and water. ''K''ow is also frequently referred to by the symbol P, especially in the English literature. It is also called ''n''-octanol-water partition ratio. ''K''ow serves as a measure of the relationship between lipophilicity (fat solubility) and hydrophilicity (water solubility) of a substance. The value is greater than one if a substance is more soluble in fat-like solvents such as n-octanol, and less than one if it is more soluble in water. If a substance is present as several chemical species in the octanol-water system due to association or dissociation, each species is assigned its own ''K''ow value. A related value, D, does not distinguish between different species, only indicating the concentration ratio of the substance between the two phases. History In 1899, Charles Ernest Overton and Hans Horst Meyer independently proposed t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioaccumulation
Bioaccumulation is the gradual accumulation of substances, such as pesticides or other chemicals, in an organism. Bioaccumulation occurs when an organism absorbs a substance at a rate faster than that at which the substance is lost or eliminated by catabolism and excretion. Thus, the longer the biological half-life of a toxic substance, the greater the risk of chronic poisoning, even if environmental levels of the toxin are not very high. Bioaccumulation, for example in fish, can be predicted by models. Hypothesis for molecular size cutoff criteria for use as bioaccumulation potential indicators are not supported by data. Biotransformation can strongly modify bioaccumulation of chemicals in an organism. Toxicity induced by metals is associated with bioaccumulation and biomagnification. Storage or uptake of metals faster than the rate at which an organism metabolizes and excretes lead to the accumulation of that metal. The presence of various chemicals and harmful substances i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell (biology)
The cell is the basic structural and functional unit of life forms. Every cell consists of a cytoplasm enclosed within a membrane, and contains many biomolecules such as proteins, DNA and RNA, as well as many small molecules of nutrients and metabolites.Cell Movements and the Shaping of the Vertebrate Body in Chapter 21 of Molecular Biology of the Cell '' fourth edition, edited by Bruce Alberts (2002) published by Garland Science. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos. It is also common to describe small molecules such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base ( adenine), the sugar ribose, and the triphosphate. Structure ATP consists of an adenine attached by the 9-nitrogen atom to the 1′ carbon atom of a sugar ( ribose), which in tu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Xylene Sulfonate
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. They are slippery to the touch, can taste bitter and change the color of pH indicators (e.g., turn red litmus paper blue). In water, by altering the autoionization equilibrium, bases yield solutions in which the hydrogen ion activity is lower than it is in pure water, i.e., the water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonic Acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: :RC6H5 + SO3 -> RC6H4SO3H In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. Alkylsulfonic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carl Neuberg
Carl Alexander Neuberg (29 July 1877 – 30 May 1956) was an early pioneer in biochemistry, and he is often referred to as the "father of modern biochemistry". His notable contribution to science includes the discovery of the carboxylase and the elucidation of alcoholic fermentation which he showed to be a process of successive enzymatic steps, an understanding that became crucial as to how metabolic pathways would be investigated by later researchers. Personal life Carl Sandel Neuberg was born on 29 July 1877 to a Jewish family in Hanover as the first child of Julius and Alma Neuberg. He was educated in the classical language gymnasium Lyceum I of the Ratsgymnasium until he was 15. In 1892 he moved with his parents to Berlin where he attended Friedrich-Werdersches Gymnasium. After graduating school in 1896, he studied astronomy, but soon switched to chemistry to comply with his father's wishes for him to become a master of brewery. He studied at the University of Würzburg and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |