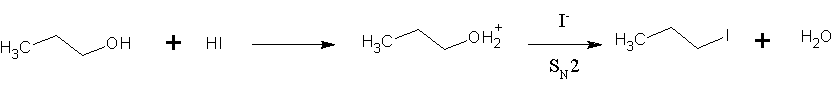|
Hofmann Elimination
Hofmann elimination is an elimination reaction of an amine to form alkenes. The least stable alkene (the one with the least number of substituents on the carbons of the double bond), called the Hofmann product, is formed. This tendency, known as the Hofmann alkene synthesis rule, is in contrast to usual elimination reactions, where Zaitsev's rule predicts the formation of the most stable alkene. It is named after its discoverer, August Wilhelm von Hofmann. The reaction starts with the formation of a quaternary ammonium iodide salt by treatment of the amine with excess methyl iodide (exhaustive methylation), followed by treatment with silver oxide and water to form a quaternary ammonium hydroxide. When this salt is decomposed by heat, the Hofmann product is preferentially formed due to the steric bulk of the leaving group causing the hydroxide to abstract the more easily accessible hydrogen. : In the Hofmann elimination, the least substituted alkene is typically favored due t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
August Wilhelm Von Hofmann
August Wilhelm von Hofmann (8 April 18185 May 1892) was a German chemist who made considerable contributions to organic chemistry. His research on aniline helped lay the basis of the aniline-dye industry, and his research on coal tar laid the groundwork for his student Charles Mansfield's practical methods for extracting benzene and toluene and converting them into nitro compounds and amines. Hofmann's discoveries include formaldehyde, hydrazobenzene, the isonitriles, and allyl alcohol. He prepared three ethylamines and tetraethylammonium compounds and established their structural relationship to ammonia. After studying under Justus von Liebig at the University of Giessen, Hofmann became the first director of the Royal College of Chemistry, now part of Imperial College London, in 1845. In 1865 he returned to Germany to accept a position at the University of Berlin as a teacher and researcher. After his return he co-founded the German Chemical Society (''Deutsche Chemis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cope Elimination
The Cope reaction or Cope elimination, developed by Arthur C. Cope, is an elimination reaction of the N-oxide to form an alkene and a hydroxylamine. Mechanism and applications The reaction mechanism involves an intramolecular 5-membered cyclic transition state, leading to a ''syn'' elimination product, an Ei pathway. This organic reaction is closely related to the Hofmann elimination, but the base is a part of the leaving group. The amine oxide is prepared by oxidation of the corresponding amine with an oxidant such as meta-chloroperoxybenzoic acid (''m''CPBA). The actual elimination just requires heat. : Illustrative of the Cope reaction is a synthesis of methylenecyclohexane: : Piperidines are resistant to an intramolecular Cope reaction but with pyrrolidine and with rings of size 7 and larger, the reaction product is an unsaturated hydroxyl amine. This result is consistent with the 5-membered cyclic transition state. : Reverse reaction The reverse or retro-Cope el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elimination Reactions
Elimination may refer to: Science and medicine *Elimination reaction, an organic reaction in which two functional groups split to form an organic product *Bodily waste elimination, discharging feces, urine, or foreign substances from the body via defecation, urination, and emesis *Drug elimination, clearance of a drug or other foreign agent from the body *Elimination, the destruction of an infectious disease in one region of the world as opposed to its eradication from the entire world *Hazard elimination, the most effective type of hazard control * Elimination (pharmacology), processes by which a drug is eliminated from an organism Logic and mathematics * Elimination theory, the theory of the methods to eliminate variables between polynomial equations. * Disjunctive syllogism, a rule of inference * Gaussian elimination, a method of solving systems of linear equations * Fourier–Motzkin elimination, an algorithm for reducing systems of linear inequalities * Process of e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emde Degradation
The Emde degradation (also called Emde-reaction or Emde-reduction) is a method for the reduction of a quaternary ammonium cation to a tertiary amine with sodium amalgam:{{cite book , first1=M. B. , last1=Smith , first2=J. , last2=March , title=March's Advanced Organic Chemistry , publisher=Wiley , year=2001 , isbn=0-471-58589-0 This organic reaction was first described in 1909 by the German chemist Hermann Emde and was for a long time of great importance in structure elucidation of many alkaloids, for example that of ephedrine. Alternative reducing agents exist for this reaction; for instance, lithium aluminium hydride. See also * Related reactions are the Hofmann elimination and the von Braun reaction The von Braun reaction is an organic reaction in which a tertiary amine reacts with cyanogen bromide to an organocyanamide. An example is the reaction of ''N'',''N''-dimethyl-1-naphthylamine: These days, most chemist have replaced cyanogen ... References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berichte Der Deutschen Chemischen Gesellschaft
''Chemische Berichte'' (usually abbreviated as ''Ber.'' or ''Chem. Ber.'') was a German-language scientific journal of all disciplines of chemistry founded in 1868. It was one of the oldest scientific journals in chemistry, until it merged with '' Recueil des Travaux Chimiques des Pays-Bas'' to form ''Chemische Berichte/Recueil'' in 1997. ''Chemische Berichte/Recueil'' was then merged with other European journals in 1998 to form ''European Journal of Inorganic Chemistry''. History Founded in 1868 as ''Berichte der Deutschen Chemischen Gesellschaft'' (, CODEN BDCGAS), it operated under this title until 1928 (Vol. 61). The journal was then split into: * ''Berichte der Deutschen Chemischen Gesellschaft, A: Vereins-Nachrichten'' (, CODEN BDCAAS), and * ''Berichte der Deutschen Chemischen Gesellschaft, B: Abhandlungen'' (, CODEN BDCBAD). Vol. 78 and 79 (1945–1946) were omitted and not published due to World War II. The journal was renamed ''Chemische Berichte'' (, CODEN CHBEAM) in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Iodide
Hydrogen iodide () is a diatomic molecule and hydrogen halide. Aqueous solutions of HI are known as hydroiodic acid or hydriodic acid, a strong acid. Hydrogen iodide and hydroiodic acid are, however, different in that the former is a gas under standard conditions, whereas the other is an aqueous solution of the gas. They are interconvertible. HI is used in organic and inorganic synthesis as one of the primary sources of iodine and as a reducing agent. Properties of hydrogen iodide HI is a colorless gas that reacts with oxygen to give water and iodine. With moist air, HI gives a mist (or fumes) of hydroiodic acid. It is exceptionally soluble in water, giving hydroiodic acid. One liter of water will dissolve 425 liters of HI gas, the most concentrated solution having only four water molecules per molecule of HI. Hydroiodic acid Hydroiodic acid is not pure hydrogen iodide, but a mixture containing it. Commercial "concentrated" hydroiodic acid usually contains 48–57% HI by ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Test
In chemistry, a chemical test is a qualitative or quantitative procedure designed to identify, quantify, or characterise a chemical compound or chemical group. Purposes Chemical testing might have a variety of purposes, such as to: * Determine if, or verify that, the requirements of a specification, regulation, or contract are met * Decide if a new product development program is on track: Demonstrate proof of concept * Demonstrate the utility of a proposed patent * Determine the interactions of a sample with other known substances * Determine the composition of a sample * Provide standard data for other scientific, medical, and Quality assurance functions * Validate suitability for end-use * Provide a basis for Technical communication * Provide a technical means of comparison of several options * Provide evidence in legal proceedings Biochemical tests * Clinistrips quantitatively test for sugar in urine * The Kastle-Meyer test tests for the presence of blood * Salicyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver Nitrate
Silver nitrate is an inorganic compound with chemical formula . It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called ''lunar caustic'' because silver was called ''luna'' by ancient alchemists who associated silver with the moon. In solid silver nitrate, the silver ions are three- coordinated in a trigonal planar arrangement. Synthesis and structure Albertus Magnus, in the 13th century, documented the ability of nitric acid to separate gold and silver by dissolving the silver. Indeed silver nitrate can be prepared by dissolving silver in nitric acid followed by evaporation of the solution. The stoichiometry of the reaction depends upon the concentration of nitric acid used. :3 Ag + 4 HNO3 (cold and diluted) → 3 AgNO3 + 2 H2O + NO :Ag + 2 HNO3 (hot and concentrated) → AgNO3 + H2O + NO2 The structure of silver nitrate has been examined by X-ray crystallography ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as '' ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Trap
In chemistry, a chemical trap is a chemical compound that is used to detect unstable compounds. The method relies on efficiency of bimolecular reactions with reagents to produce a more easily characterize trapped product. In some cases, the trapping agent is used in large excess. Case studies Cyclobutadiene A famous example is the detection of cyclobutadiene released upon oxidation of cyclobutadieneiron tricarbonyl. When this degradation is conducted in the presence of an alkyne, the cyclobutadiene is trapped as a bicyclohexadiene. The requirement for this trapping experiment is that the oxidant (ceric ammonium nitrate) and the trapping agent be mutually compatible. : Diphosphorus Diphosphorus is an old target of chemists since it is the heavy analogue of N2. Its fleeting existence is inferred by the controlled degradation of certain niobium complexes in the presence of trapping agents. Again, a Diels-Alder strategy is employed in the trapping: : Silylene Another classic but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trans-cyclooctene
''trans''-Cyclooctene is a cyclic hydrocarbon with the formula ��(CH2)6CH=CH– where the two C–C single bonds adjacent to the double bond are on opposite sides of the latter's plane. It is a colorless liquid with a disagreeable odor. Cyclooctene is notable as the smallest cycloalkene that is readily isolated as its ''trans''- isomer. The ''cis''-isomer is much more stable; the ring-strain energies being 16.7 and 7.4 kcal/mol, respectively.Ron Walker, Rosemary M. Conrad, and Robert H. Grubbs (2009): "The living ROMP of ''trans''-cyclooctene". ''Macromolecules'', volume 42, issue 3, pages 599–605. A planar arrangement of the ring carbons would be too strained, and therefore the stable conformations of the ''trans'' form have a bent (non-planar) ring. Computations indicate that the most stable "crown" conformation has the carbon atoms alternately above and below the plane of the ring. A "half-chair" conformation, with about 6 kcal/mol higher energy, has carbons 2,3,5, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |