|
Haematoxylin
Haematoxylin or hematoxylin (), also called natural black 1 or C.I. 75290, is a compound extracted from heartwood of the logwood tree (''Haematoxylum campechianum'') with a chemical formula of . This naturally derived dye has been used as a histologic stain, ink and as a dye in the textile and leather industry. As a dye, haematoxylin has been called Palo de Campeche, logwood extract, bluewood and blackwood. In histology, haematoxylin staining is commonly followed ( counterstained), with eosin, when paired, this staining procedure is known as H&E staining, and is one of the most commonly used combinations in histology. In addition to its use in the H&E stain, haematoxylin is also a component of the Papanicolaou stain (or PAP stain) which is widely used in the study of cytology specimens. Although the stain is commonly called ''haematoxylin'', the active colourant is the oxidized form haematein, which forms strongly coloured complexes with certain metal ions (commonly Fe(III) an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
H&E Stain
Hematoxylin and eosin stain ( or haematoxylin and eosin stain or hematoxylin-eosin stain; often abbreviated as H&E stain or HE stain) is one of the principal tissue stains used in histology. It is the most widely used stain in medical diagnosis and is often the gold standard. For example, when a pathologist looks at a biopsy of a suspected cancer, the histological section is likely to be stained with H&E. H&E is the combination of two histological stains: hematoxylin and eosin. The hematoxylin stains cell nuclei a purplish blue, and eosin stains the extracellular matrix and cytoplasm pink, with other structures taking on different shades, hues, and combinations of these colors. Hence a pathologist can easily differentiate between the nuclear and cytoplasmic parts of a cell, and additionally, the overall patterns of coloration from the stain show the general layout and distribution of cells and provides a general overview of a tissue sample's structure. Thus, pattern r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
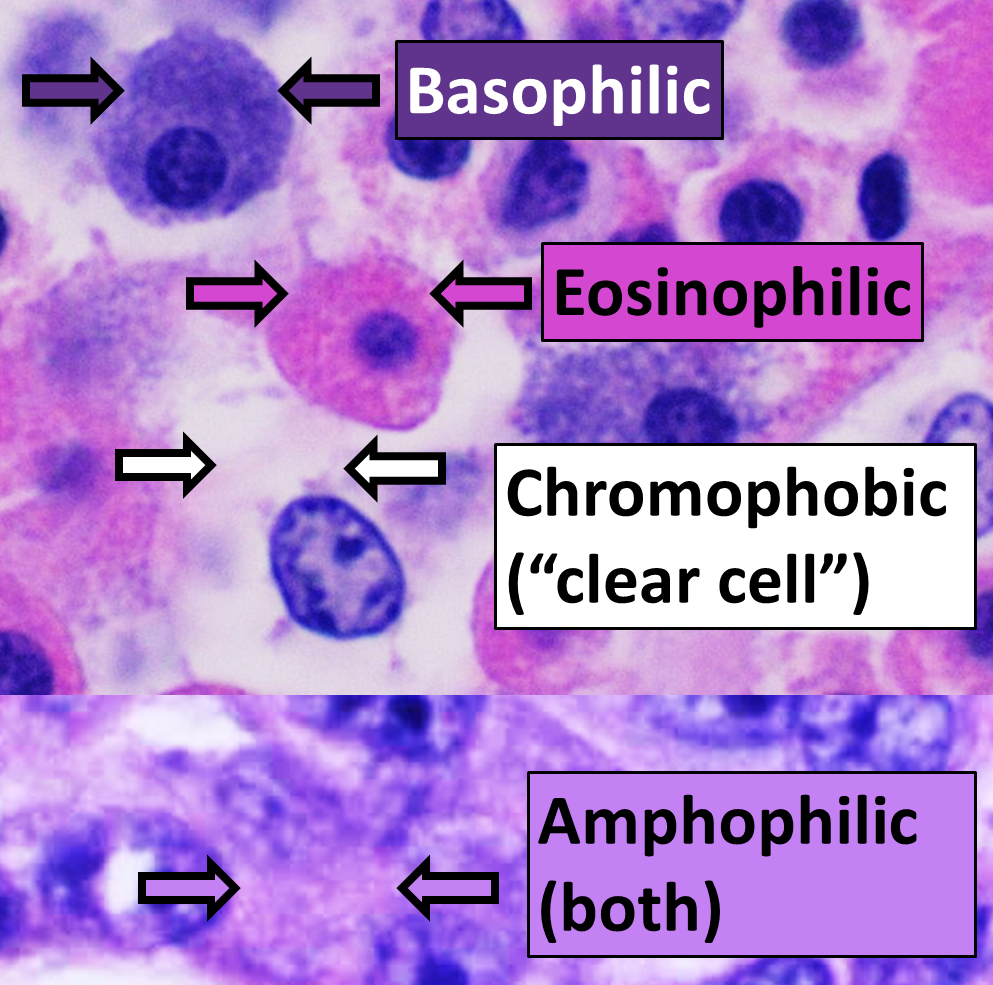
Staining
Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology (microscopic study of biological tissues), in cytology (microscopic study of cells), and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues (highlighting, for example, muscle fibers or connective tissue), cell populations (classifying different blood cells), or organelles within individual cells. In biochemistry, it involves adding a class-specific ( DNA, proteins, lipids, carbohydrates) dye to a substrate to qualify or quantify the presence of a specific compound. Staining and fluorescent tagging can serve similar purposes. Biological staining is also used to mark cells in flow cytometry, and to flag proteins or nucleic acids in gel electrophoresis. Light microscopes are us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eosin
Eosin is the name of several fluorescent acidic compounds which bind to and form salts with basic, or eosinophilic, compounds like proteins containing amino acid residues such as arginine and lysine, and stains them dark red or pink as a result of the actions of bromine on eosin. In addition to staining proteins in the cytoplasm, it can be used to stain collagen and muscle fibers for examination under the microscope. Structures, that stain readily with eosin, are termed eosinophilic. In the field of histology, Eosin Y is the form of eosin used most often as a histologic stain. Etymology Eosin was named by its inventor Heinrich Caro after the nickname ( Eos) of a childhood friend, Anna Peters. Variants There are actually two very closely related compounds commonly referred to as eosin. Most often used is in histology is Eosin Y (also known as eosin Y ws, eosin yellowish, Acid Red 87, C.I. 45380, bromoeosine, bromofluoresceic acid, D&C Red No. 22); it has a very slightly yell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Papanicolaou Stain
Papanicolaou stain (also Papanicolaou's stain and Pap stain) is a multichromatic (multicolored) cytological staining technique developed by George Papanicolaou in 1942. The Papanicolaou stain is one of the most widely used stains in cytology, where it is used to aid pathologists in making a diagnosis. Although most notable for its use in the detection of cervical cancer in the Pap test or Pap smear, it is also used to stain non-gynecological specimen preparations from a variety of bodily secretions and from small needle biopsies of organs and tissues. Papanicolaou published three formulations of this stain in 1942, 1954, and 1960. Usage Pap staining is used to differentiate cells in smear preparations (in which samples are spread or smeared onto a glass microscope slide) from various bodily secretions and needle biopsies; the specimens may include gynecological smears ( Pap smears), sputum, brushings, washings, urine, cerebrospinal fluid, abdominal fluid, pleural f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haematoxylum Campechianum
''Haematoxylum campechianum'' (blackwood, bloodwood tree, bluewood, campeachy tree, campeachy wood, campeche logwood, campeche wood, Jamaica wood, logwood or logwood tree) is a species of flowering tree in the legume family, Fabaceae, that is native to southern Mexico,where it is known as ''Árbol de campeche'', and introduced to the Caribbean, northern Central America, and other localities around the world. The tree was of great economic importance from the 17th century to the 19th century, when it was commonly logged and exported to Europe for use in dyeing fabrics. The modern nation of Belize developed from 17th- and 18th-century logging camps established by the English. The tree's scientific name means "bloodwood" (''haima'' being Greek for blood and ''xylon'' for wood). Uses ''Haematoxylum campechianum'' was used for a long time as a natural source of dye. The woodchips are still used as an important source of haematoxylin, which is used in histology for staining ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haematein
Hematein (US spelling) or haematein is an oxidized derivative of haematoxylin, used in staining. Haematein should not be confused with haematin, which is a brown to black iron-containing pigment formed by decomposition of haemoglobin. In the Colour Index (but nowhere else), haematein is called haematine. Properties Hematein exhibits indicator-like properties, being blue and less soluble in aqueous alkaline conditions, and red and more soluble in alcoholic acidic conditions. Dissolved haematein slowly reacts with atmospheric oxygen, yielding products that have not found applications. Applications In acidic solutions, complexes of hematein with metals (usually aluminium or iron, but also chromium, zirconium and several others) are used as biological stains. Aluminium-haematein (haemalum) is the "routine" stain for cell nuclei in sections of human and other animal tissues. Metal-haematein stains are available also for objects other than nuclei, including myelin sheaths of nerve fibr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Counterstain
A counterstain is a stain with colour contrasting to the principal stain, making the stained structure easily visible using a microscope. Examples include the malachite green counterstain to the fuchsine stain in the Gimenez staining technique and the eosin counterstain to haematoxylin in the H&E stain. In Gram staining, crystal violet stains only Gram-positive bacteria, and safranin counterstain is applied which stains all cells, allowing the identification of Gram-negative bacteria as well. An alternative method uses dilute carbofluozide. Counterstains are sometimes used to separate animals from organic detritus In biology, detritus () is dead particulate organic material, as distinguished from dissolved organic material. Detritus typically includes the bodies or fragments of bodies of dead organisms, and fecal material. Detritus typically hosts commun ... in microbiology studies. References Staining {{pathology-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biological Stain Commission
The Biological Stain Commission (BSC) is an organization that provides third-party testing and certification of dyes and a few other compounds that are used to enhance contrast in specimens examined in biological and medical laboratories. The BSC is a 95-year-old organization well known to many thousands of scientists, worldwide but especially in N America, who buy BSC-certified stains for staining Microscopy, microscopic preparations and for making selective Growth medium, culture media for bacteria. Manufacturers and other vendors submit samples from their batches of dyes to the BSC's independent laboratory in Rochester NY. The BSC's certification label on a bottle of dye indicates that the contents are from a batch that passed the tests for chemical purity and for efficacy as a biological stain. These tests are published (Penney et al. 2002a). Changes to tests and additions to the list of stains eligible for certification are published from time to time in ''Biotechnic & Histoch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pap Test
The Papanicolaou test (abbreviated as Pap test, also known as Pap smear (AE), cervical smear (BE), cervical screening (BE), or smear test (BE)) is a method of cervical screening used to detect potentially precancerous and cancerous processes in the cervix (opening of the uterus or womb) or colon (in both men and women). Abnormal findings are often followed up by more sensitive diagnostic procedures and, if warranted, interventions that aim to prevent progression to cervical cancer. The test was independently invented in the 1920s by Georgios Papanikolaou and Aurel Babeș and named after Papanikolaou. A simplified version of the test was introduced by Anna Marion Hilliard in 1957. A Pap smear is performed by opening the vagina with a speculum and collecting cells at the outer opening of the cervix at the transformation zone (where the outer squamous cervical cells meet the inner glandular endocervical cells), using an Ayre spatula or a cytobrush. A similar method is used to col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cervical Cancer
Cervical cancer is a cancer arising from the cervix. It is due to the abnormal growth of cells that have the ability to invade or spread to other parts of the body. Early on, typically no symptoms are seen. Later symptoms may include abnormal vaginal bleeding, pelvic pain or pain during sexual intercourse. While bleeding after sex may not be serious, it may also indicate the presence of cervical cancer. Human papillomavirus infection (HPV) causes more than 90% of cases; most women who have had HPV infections, however, do not develop cervical cancer. HPV 16 and 18 strains are responsible for nearly 50% of high grade cervical pre-cancers. Other risk factors include smoking, a weak immune system, birth control pills, starting sex at a young age, and having many sexual partners, but these are less important. Genetic factors also contribute to cervical cancer risk. Cervical cancer typically develops from precancerous changes over 10 to 20 years. About 90% of cervical cancer ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
American And British English Spelling Differences
Despite the various English dialects spoken from country to country and within different regions of the same country, there are only slight regional variations in English orthography, the two most notable variations being British and American spelling. Many of the differences between American and British English date back to a time before spelling standards were developed. For instance, some spellings seen as "American" today were once commonly used in Britain, and some spellings seen as "British" were once commonly used in the United States. A "British standard" began to emerge following the 1755 publication of Samuel Johnson's '' A Dictionary of the English Language'', and an "American standard" started following the work of Noah Webster and, in particular, his ''An American Dictionary of the English Language'', first published in 1828. Webster's efforts at spelling reform were somewhat effective in his native country, resulting in certain well-known patterns of spelling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |