|
Freshwater
Fresh water or freshwater is any naturally occurring liquid or frozen water containing low concentrations of dissolved salts and other total dissolved solids. Although the term specifically excludes seawater and brackish water, it does include non- salty mineral-rich waters such as chalybeate springs. Fresh water may encompass frozen and meltwater in ice sheets, ice caps, glaciers, snowfields and icebergs, natural precipitations such as rainfall, snowfall, hail/ sleet and graupel, and surface runoffs that form inland bodies of water such as wetlands, ponds, lakes, rivers, streams, as well as groundwater contained in aquifers, subterranean rivers and lakes. Fresh water is the water resource that is of the most and immediate use to humans. Water is critical to the survival of all living organisms. Many organisms can thrive on salt water, but the great majority of higher plants and most insects, amphibians, reptiles, mammals and birds need fresh water to survive. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wetland
A wetland is a distinct ecosystem that is flooded or saturated by water, either permanently (for years or decades) or seasonally (for weeks or months). Flooding results in oxygen-free (Anoxic waters, anoxic) processes prevailing, especially in the soils. The primary factor that distinguishes wetlands from terrestrial land forms or Body of water, water bodies is the characteristic vegetation of aquatic plants, adapted to the unique anoxic hydric soils. Wetlands are considered among the most biologically diverse of all ecosystems, serving as home to a wide range of plant and animal species. Methods for assessing wetland functions, wetland ecological health, and general wetland condition have been developed for many regions of the world. These methods have contributed to wetland conservation partly by raising public awareness of the functions some wetlands provide. Wetlands occur naturally on every continent. The water in wetlands is either freshwater, brackish or seawater, saltwate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
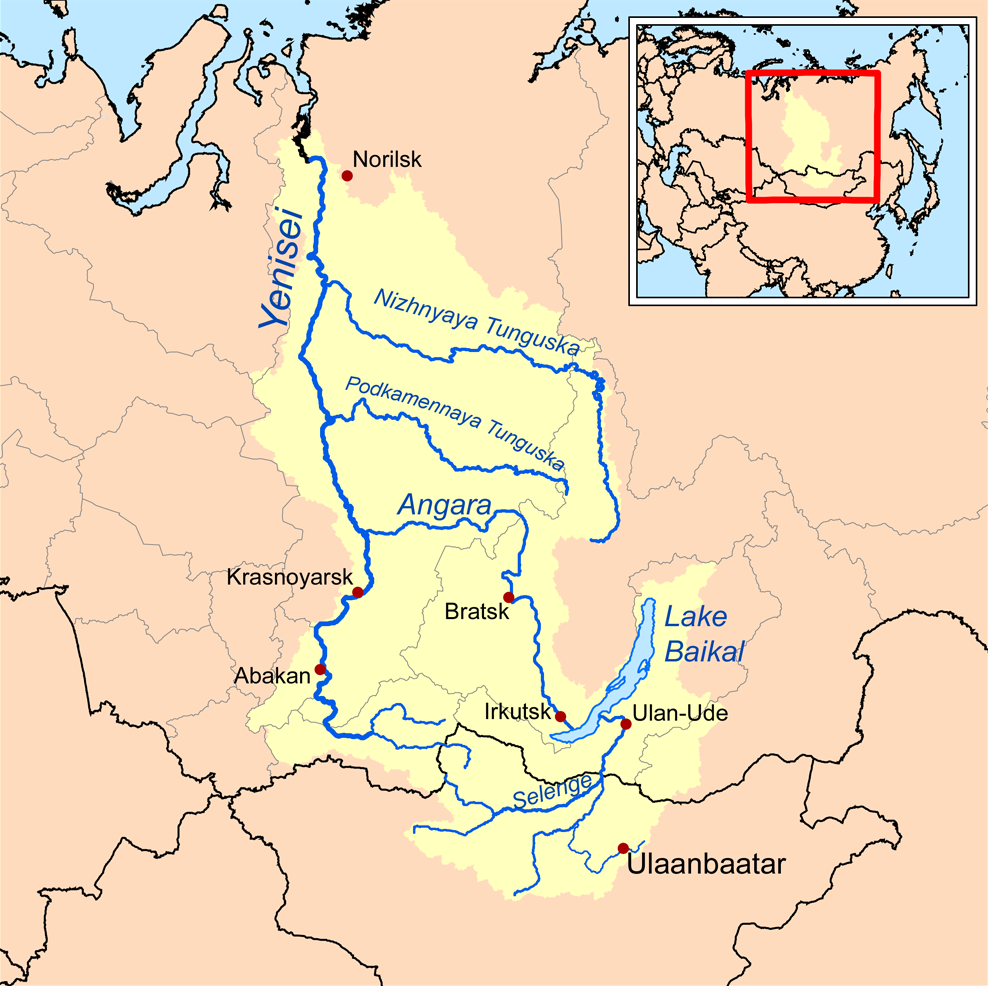
Lake Baikal
Lake Baikal (, russian: Oзеро Байкал, Ozero Baykal ); mn, Байгал нуур, Baigal nuur) is a rift lake in Russia. It is situated in southern Siberia, between the federal subjects of Irkutsk Oblast to the northwest and the Republic of Buryatia to the southeast. With of water, Lake Baikal is the world's largest freshwater lake by volume, containing 22–23% of the world's fresh surface water, more than all of the North American Great Lakes combined. It is also the world's deepest lake, with a maximum depth of , and the world's oldest lake, at 25–30 million years. At —slightly larger than Belgium—Lake Baikal is the world's seventh-largest lake by surface area. It is among the world's clearest lakes. Lake Baikal is home to thousands of species of plants and animals, many of them endemic to the region. It is also home to Buryat tribes, who raise goats, camels, cattle, sheep, and horses on the eastern side of the lake, where the mean tempera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brackish Water
Brackish water, sometimes termed brack water, is water occurring in a natural environment that has more salinity than freshwater, but not as much as seawater. It may result from mixing seawater (salt water) and fresh water together, as in estuaries, or it may occur in brackish fossil aquifers. The word comes from the Middle Dutch root '' brak''. Certain human activities can produce brackish water, in particular civil engineering projects such as dikes and the flooding of coastal marshland to produce brackish water pools for freshwater prawn farming. Brackish water is also the primary waste product of the salinity gradient power process. Because brackish water is hostile to the growth of most terrestrial plant species, without appropriate management it is damaging to the environment (see article on shrimp farms). Technically, brackish water contains between 0.5 and 30 grams of salt per litre—more often expressed as 0.5 to 30 parts per thousand (‰), which is a specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amazon River
The Amazon River (, ; es, Río Amazonas, pt, Rio Amazonas) in South America is the largest river by discharge volume of water in the world, and the disputed longest river system in the world in comparison to the Nile. The headwaters of the Apurímac River on Nevado Mismi had been considered for nearly a century as the Amazon basin's most distant source, until a 2014 study found it to be the headwaters of the Mantaro River on the Cordillera Rumi Cruz in Peru. The Mantaro and Apurímac rivers join, and with other tributaries form the Ucayali River, which in turn meets the Marañón River upstream of Iquitos, Peru, forming what countries other than Brazil consider to be the main stem of the Amazon. Brazilians call this section the Solimões River above its confluence with the Rio Negro forming what Brazilians call the Amazon at the Meeting of Waters ( pt, Encontro das Águas) at Manaus, the largest city on the river. The Amazon River has an average discharge of about – ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pond
A pond is an area filled with water, either natural or artificial, that is smaller than a lake. Defining them to be less than in area, less than deep, and with less than 30% emergent vegetation helps in distinguishing their ecology from that of lakes and wetlands.Clegg, J. (1986). Observer's Book of Pond Life. Frederick Warne, London Ponds can be created by a wide variety of natural processes (e.g. on floodplains as cutoff river channels, by glacial processes, by peatland formation, in coastal dune systems, by beavers), or they can simply be isolated depressions (such as a kettle hole, vernal pool, prairie pothole, or simply natural undulations in undrained land) filled by runoff, groundwater, or precipitation, or all three of these. They can be further divided into four zones: vegetation zone, open water, bottom mud and surface film. The size and depth of ponds often varies greatly with the time of year; many ponds are produced by spring flooding from rivers. Ponds may b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lake
A lake is an area filled with water, localized in a basin, surrounded by land, and distinct from any river or other outlet that serves to feed or drain the lake. Lakes lie on land and are not part of the ocean, although, like the much larger oceans, they do form part of the Earth's water cycle. Lakes are distinct from lagoons, which are generally coastal parts of the ocean. Lakes are typically larger and deeper than ponds, which also lie on land, though there are no official or scientific definitions. Lakes can be contrasted with rivers or streams, which usually flow in a channel on land. Most lakes are fed and drained by rivers and streams. Natural lakes are generally found in mountainous areas, rift zones, and areas with ongoing glaciation. Other lakes are found in endorheic basins or along the courses of mature rivers, where a river channel has widened into a basin. Some parts of the world have many lakes formed by the chaotic drainage patterns left over from the last ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food, energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. "Water" is also the name of the liquid state of H2O at standard temperature and pressure. A number of natural states of water exist. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iceberg
An iceberg is a piece of freshwater ice more than 15 m long that has broken off a glacier or an ice shelf and is floating freely in open (salt) water. Smaller chunks of floating glacially-derived ice are called "growlers" or "bergy bits". The sinking of the ''Titanic'' in 1912 led to the formation of the International Ice Patrol in 1914. Much of an iceberg is below the surface, which led to the expression " tip of the iceberg" to illustrate a small part of a larger unseen issue. Icebergs are considered a serious maritime hazard. Icebergs vary considerably in size and shape. Icebergs that calve from glaciers in Greenland are often irregularly shaped while Antarctic ice shelves often produce large tabular (table top) icebergs. The largest iceberg in recent history (2000), named B-15, measured nearly 300 km × 40 km. The largest iceberg on record was an Antarctic tabular iceberg of over [] sighted west of Scott Island, in the South Pacific Ocean, by the USS Glacie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Everglades
The Everglades is a natural region of tropical wetlands in the southern portion of the U.S. state of Florida, comprising the southern half of a large drainage basin within the Neotropical realm. The system begins near Orlando with the Kissimmee River, which discharges into the vast but shallow Lake Okeechobee. Water leaving the lake in the wet season forms a slow-moving river wide and over long, flowing southward across a limestone shelf to Florida Bay at the southern end of the state. The Everglades experiences a wide range of weather patterns, from frequent flooding in the wet season to drought in the dry season. Throughout the 20th century, the Everglades suffered significant loss of habitat and environmental degradation. Human habitation in the southern portion of the Florida peninsula dates to 15,000 years ago. Before European colonization, the region was dominated by the native Calusa and Tequesta tribes. With Spanish colonization, both tribes declined gradually ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Dissolved Solids
Total dissolved solids (TDS) is a measure of the dissolved combined content of all inorganic and organic substances present in a liquid in molecular, ionized, or micro-granular ( colloidal sol) suspended form. TDS concentrations are often reported in parts per million (ppm). Water TDS concentrations can be determined using a digital meter. Generally, the operational definition is that the solids must be small enough to survive filtration through a filter with 2-micrometer (nominal size, or smaller) pores. Total dissolved solids are normally discussed only for freshwater systems, as salinity includes some of the ions constituting the definition of TDS. The principal application of TDS is in the study of water quality for streams, rivers, and lakes. Although TDS is not generally considered a primary pollutant (e.g. it is not deemed to be associated with health effects), it is used as an indication of aesthetic characteristics of drinking water and as an aggregate indicator of the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately of dissolved salts (predominantly sodium () and chloride () ions). The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water and pure water (density 1.0 kg/L at ) because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about . The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was . Seawater pH is typically limited to a range between 7.5 and 8.4. However, there is no universally accepted reference pH-scale for seawater and the difference between measure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glacier
A glacier (; ) is a persistent body of dense ice that is constantly moving under its own weight. A glacier forms where the accumulation of snow exceeds its ablation over many years, often centuries. It acquires distinguishing features, such as crevasses and seracs, as it slowly flows and deforms under stresses induced by its weight. As it moves, it abrades rock and debris from its substrate to create landforms such as cirques, moraines, or fjords. Although a glacier may flow into a body of water, it forms only on land and is distinct from the much thinner sea ice and lake ice that form on the surface of bodies of water. On Earth, 99% of glacial ice is contained within vast ice sheets (also known as "continental glaciers") in the polar regions, but glaciers may be found in mountain ranges on every continent other than the Australian mainland, including Oceania's high-latitude oceanic island countries such as New Zealand. Between latitudes 35°N and 35°S, glaciers occur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |







.jpg)