|
Formetanate
Formetanate is an insecticide and acaricide. It is used on alfalfa grown for seed and on some fruits, including citrus, pome, and stone fruit In botany, a drupe (or stone fruit) is an indehiscent fruit in which an outer fleshy part ( exocarp, or skin, and mesocarp, or flesh) surrounds a single shell (the ''pit'', ''stone'', or ''pyrena'') of hardened endocarp with a seed (''kernel' ...s. Pesticide Management Education Program See also * FormparanateExternal links *References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylcholinesterase Inhibitors
Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family. Acetylcholinesterase inhibitors are classified as reversible, irreversible, or quasi-irreversible (also called pseudo-irreversible). Mechanism of action Organophosphates Organophosphates like TEPP and sarin inhibit cholinesterases, enzymes that hydrolyze the neurotransmitter acetylcholine. The active centre of cholinesterases feature two important sites, namely th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formparanate
Formparanate (chemical formula: C12H17N3O2) is a chemical compound used in acaricides and insecticides. See also * Formetanate Formetanate is an insecticide and acaricide. It is used on alfalfa grown for seed and on some fruits, including citrus, pome, and stone fruit In botany, a drupe (or stone fruit) is an indehiscent fruit in which an outer fleshy part ( exo ... References {{Acetylcholine metabolism and transport modulators Acetylcholinesterase inhibitors Carbamate insecticides Amidines Acaricides Aromatic carbamates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloride
In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base (e.g. an amine). An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid. Uses Converting amines into their hydrochlorides is a common way to improve their water solubility, which can be desirable for substances used in medications. The European Pharmacopoeia lists more than 200 hydrochlorides as active ingredients in medications. These hydrochlorides, compared to free bases, may more readily dissolve in the gastrointestinal tract and be absorbed into the bloodstream more quickly. Additionally, many hydrochlorides of amines have a longer shelf-life than their respective free bases. Amine hydrochlorides represent latent forms of a more reactive free base. In this regard, formation of an amine hydrochloride confers protection. This eff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to be a major factor behind the increase in the 20th-century's agricultural productivity. Nearly all insecticides have the potential to significantly alter ecosystems; many are toxic to humans and/or animals; some become concentrated as they spread along the food chain. Insecticides can be classified into two major groups: systemic insecticides, which have residual or long term activity; and contact insecticides, which have no residual activity. The mode of action describes how the pesticide kills or inactivates a pest. It provides another way of classifying insecticides. Mode of action can be important in understanding whether an insecticide will be toxic to unrelated species, such as fish, birds and mammals. Insecticides may be repellent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acaricide
Acaricides are pesticides that kill members of the arachnid subclass ''Acari'', which includes ticks and mites. Acaricides are used both in medicine and agriculture, although the desired selective toxicity differs between the two fields. Terminology More specific words are sometimes used, depending upon the targeted group: * "Ixodicides" are substances that kill ticks. * "Miticides" are substances that kill mites. *The term scabicide is more narrow, and refers to agents specifically targeting ''Sarcoptes''. *The term "arachnicide" is more general, and refers to agents that target arachnids. This term is used much more rarely, but occasionally appears in informal writing. As a practical matter, mites are a paraphyletic grouping, and mites and ticks are usually treated as a single group. Examples Examples include: * Permethrin can be applied as a spray. The effects are not limited to mites: lice, cockroaches, fleas, mosquitos, and other insects will be affected. * Ivermectin can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alfalfa
Alfalfa () (''Medicago sativa''), also called lucerne, is a perennial flowering plant in the legume family Fabaceae. It is cultivated as an important forage crop in many countries around the world. It is used for grazing, hay, and silage, as well as a green manure and cover crop. The name alfalfa is used in North America. The name lucerne is the more commonly used name in the United Kingdom, South Africa, Australia, and New Zealand. The plant superficially resembles clover (a cousin in the same family), especially while young, when trifoliate leaves comprising round leaflets predominate. Later in maturity, leaflets are elongated. It has clusters of small purple flowers followed by fruits spiralled in 2 to 3 turns containing 10–20 seeds. Alfalfa is native to warmer temperate climates. It has been cultivated as livestock fodder since at least the era of the ancient Greeks and Romans. Etymology The word ''alfalfa'' is a Spanish modification of the Arabic word ''al-faṣfa� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citrus
''Citrus'' is a genus of flowering plant, flowering trees and shrubs in the rue family, Rutaceae. Plants in the genus produce citrus fruits, including important crops such as Orange (fruit), oranges, Lemon, lemons, grapefruits, pomelos, and lime (fruit), limes. The genus ''Citrus'' is native to South Asia, East Asia, Southeast Asia, Melanesia, and Australia (continent), Australia. Various citrus species have been used and domesticated by indigenous cultures in these areas since ancient times. From there its cultivation spread into Micronesia and Polynesia by the Austronesian expansion (c. 3000–1500 BCE); and to the Middle East and the Mediterranean (c. 1200 BCE) via the incense trade route, and onwards to Europe and the Americas. History Citrus plants are native to subtropical and tropical regions of Asia, Island Southeast Asia, Near Oceania, and northeastern Australia. Domestication of citrus species involved much hybridization and introgression, leaving much uncertainty ab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pome
In botany, a pome is a type of fruit produced by flowering plants in the subtribe Malinae of the family Rosaceae. Well-known pomes include the apple, pear, and quince. Etymology The word ''pome'' entered English in the late 14th century, and referred to an apple or an apple-shaped object. It derived from the Old French word for "apple": (12th century; modern French is ), which in turn derived from the Late Latin or Vulgar Latin word "apple", originally the plural of Latin "fruit", later "apple". Morphology A pome is an accessory fruit composed of one or more carpels surrounded by accessory tissue. The accessory tissue is interpreted by some specialists as an extension of the receptacle and is then referred to as "fruit cortex",Esau, K. 1977. ''Anatomy of seed plants''. John Wiley and Sons, New York. and by others as a fused hypanthium (floral cup). It is the most edible part of this fruit. The carpels of a pome are fused within the "core". Although the epicarp, mesocarp, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stone Fruit
In botany, a drupe (or stone fruit) is an indehiscent fruit in which an outer fleshy part (exocarp, or skin, and mesocarp, or flesh) surrounds a single shell (the ''pit'', ''stone'', or ''pyrena'') of hardened endocarp with a seed (''kernel'') inside. These fruits usually develop from a single carpel, and mostly from flowers with superior ovaries (polypyrenous drupes are exceptions). The definitive characteristic of a drupe is that the hard, lignified stone is derived from the ovary wall of the flower. In an aggregate fruit, which is composed of small, individual drupes (such as a raspberry), each individual is termed a drupelet, and may together form an aggregate fruit. Such fruits are often termed ''berries'', although botanists use a different definition of ''berry''. Other fleshy fruits may have a stony enclosure that comes from the seed coat surrounding the seed, but such fruits are not drupes. Flowering plants that produce drupes include coffee, jujube, mango, olive, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acaricides
Acaricides are pesticides that kill members of the arachnid subclass ''Acari'', which includes ticks and mites. Acaricides are used both in medicine and agriculture, although the desired selective toxicity differs between the two fields. Terminology More specific words are sometimes used, depending upon the targeted group: * "Ixodicides" are substances that kill ticks. * "Miticides" are substances that kill mites. *The term scabicide is more narrow, and refers to agents specifically targeting ''Sarcoptes''. *The term "arachnicide" is more general, and refers to agents that target arachnids. This term is used much more rarely, but occasionally appears in informal writing. As a practical matter, mites are a paraphyletic grouping, and mites and ticks are usually treated as a single group. Examples Examples include: * Permethrin can be applied as a spray. The effects are not limited to mites: lice, cockroaches, fleas, mosquitos, and other insects will be affected. * Ivermectin can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
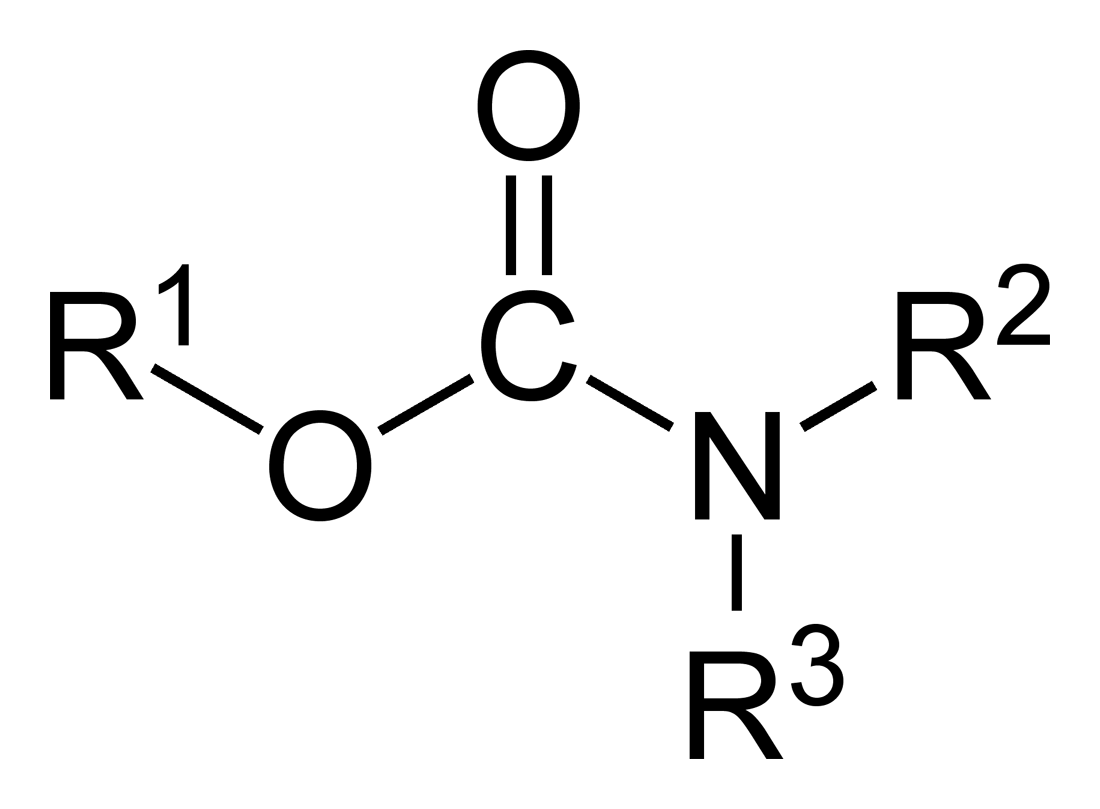
Carbamate Insecticides
In organic chemistry, a carbamate is a category of organic compounds with the general Chemical formula, formula and Chemical structure, structure , which are formally Derivative (chemistry), derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salt (chemistry), salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or salt (chemistry), ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amidines
Amidines are organic compounds with the functional group RC(NR)NR2, where the R groups can be the same or different. They are the imine derivatives of amides (RC(O)NR2). The simplest amidine is formamidine, HC(=NH)NH2. Examples of amidines include: * DBU * diminazene * benzamidine * Pentamidine * Paranyline Preparation A common route to primary amidines is the Pinner reaction. Reaction of the nitrile with alcohol in the presence of acid gives an iminoether. Treatment of the resulting compound with ammonia then completes the conversion to the amidine. Instead of using a Bronsted acid, Lewis acids such as aluminium trichloride promote the direct amination of nitriles. They are also generated by amination of an imidoyl chloride. They are also prepared by the addition of organolithium reagents to diimines, followed by protonation or alkylation. Dimethylformamide acetal reacts with primary amines to give amidines: :Me2NC(H)(OMe)2 + RNH2 → Me2NC=NHR + 2 MeOH Properties and applica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |