|
Enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). The terms ''enol'' and ''alkenol'' are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves removal of a hydrogen adjacent (α-) to the carbonyl group—i.e., deprotonation, its removal as a proton, . When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate (see images at right). The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keto-enol Tautomerism
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). The terms ''enol'' and ''alkenol'' are portmanteaus deriving from "-ene"/"alkene" and the "-ol" suffix indicating the hydroxyl group of alcohols, dropping the terminal "-e" of the first term. Generation of enols often involves removal of a hydrogen adjacent (α-) to the carbonyl group—i.e., deprotonation, its removal as a proton, . When this proton is not returned at the end of the stepwise process, the result is an anion termed an enolate (see images at right). The enolate structures shown are schematic; a more modern representation considers the molecular orbitals that are formed and occupied by electrons in the enolate. Similarly, generation of the enol often is accompanied by "trapping" or masking of the hydroxy group as an ether, such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,4-pentanedione
Acetylacetone is an organic compound with the chemical formula . It is a colorless liquid, classified as a 1,3-diketone. It exists in equilibrium with a tautomer . These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. It is a colorless liquid that is a precursor to acetylacetonate anion (commonly abbreviated acac−), a bidentate ligand. It is also a building block for the synthesis of heterocyclic compounds. Properties Tautomerism The keto and enol tautomers of acetylacetone coexist in solution. The enol form has C2v symmetry, meaning the hydrogen atom is shared equally between the two oxygen atoms. In the gas phase, the equilibrium constant, ''K''keto→enol, is 11.7, favoring the enol form. The two tautomeric forms can be distinguished by NMR spectroscopy, IR spectroscopy and other methods. The equilibrium constant tends to be high in nonpolar solvents; when k = >1, the enol form is favour ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
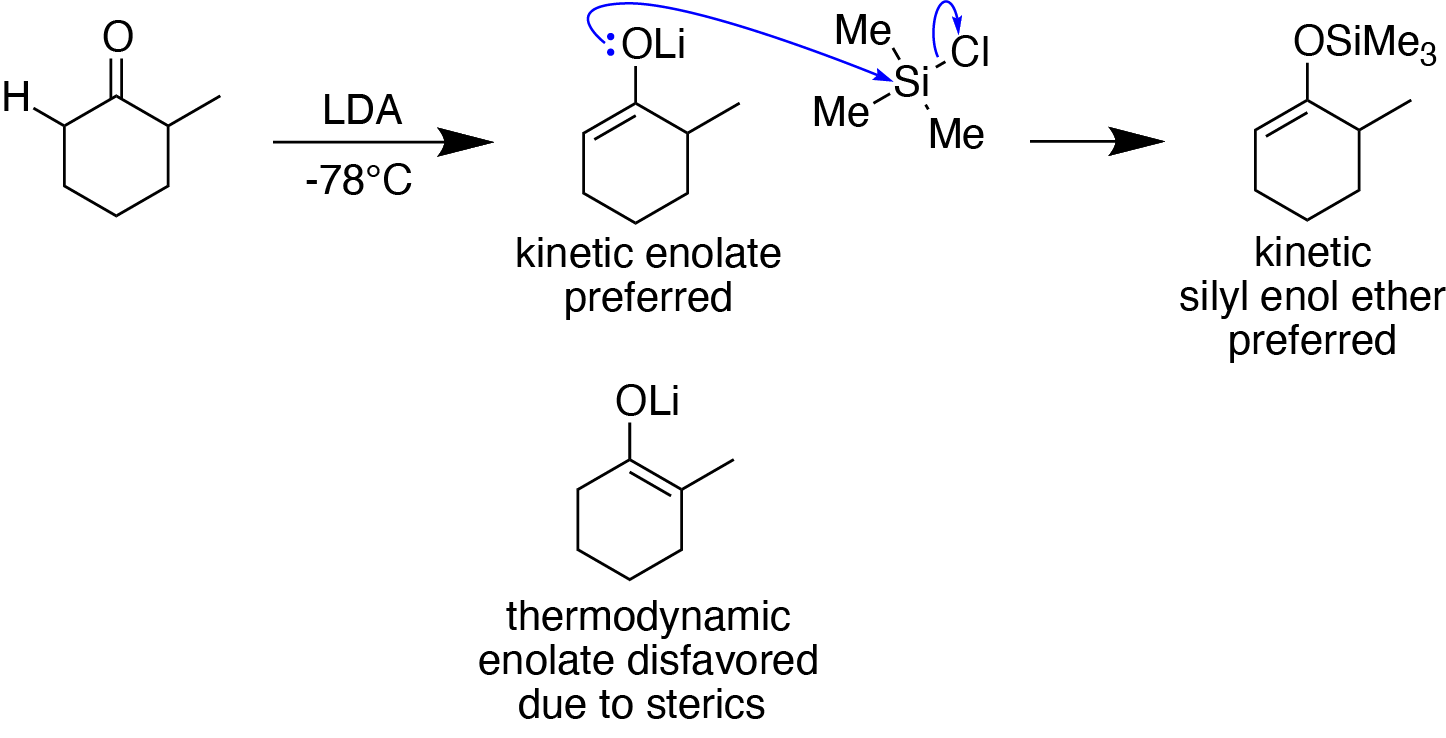
Silyl Enol Ether
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen end to an organosilicon group. They are important intermediates in organic synthesis. Synthesis Silyl enol ethers are generally prepared by reacting an enolizable Carbonyl group, carbonyl compound with a silyl electrophile and a Base (chemistry), base, or just reacting an enolate with a silyl electrophile.Clayden, J., Greeves, N., & Warren, S. (2012). Silyl enol ethers. In ''Organic chemistry'' (Second ed., pp. 466-467). Oxford University Press. Since silyl electrophiles are HSAB theory, hard and silicon-oxygen bonds are very strong, the oxygen (of the carbonyl compound or enolate) acts as the nucleophile to form a Si-O single bond. The most commonly used silyl electrophile is trimethylsilyl chloride. To increase the rate of reaction, trimethylsilyl triflate may also be used in the place of trimethylsilyl chloride as a more el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Aldehy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered ret ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tautomerism
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties, whereas resonance forms are merely alternative Lewis structure ( valence bond theory) depictions of a single chemical species, whose true structure is best described as the "average" of the idealized, hypot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
α-hydrogen
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule. Numeric locants The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of numeric prefixes to indicate the position of substituents, generally by identifying the parent hydrocarbon chain and assigning the carbon atoms based on their substituents in order of precedence. For example, there are at least two isomers of the linear form of pentanone, a ketone that contains a chain of exactly five carbon atoms. There is an oxygen atom bonded to one of the middle three carbons (if it were bonded to an end carbon, the molecule would be an aldehyde, not a ketone), but it is not clear where it is located. In this example, the carbon atoms are numbered from one to five, which starts at one end and proceeds sequentially along the chain. Now the position of the oxygen atom can be defined as on carbon atom number two, three or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were first introduced in chemical notation by Russian chemist Alexander Butlerov. Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two. Double bonds are also electron-rich, which makes them potentially more reactive in the presence of a strong electron acceptor (as in addition reactions of the halogens). File:Ethene structural.svg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |