|
Electron Pair
In chemistry, an electron pair or Lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Gilbert N. Lewis introduced the concepts of both the electron pair and the covalent bond in a landmark paper he published in 1916. Because electrons are fermions, the Pauli exclusion principle forbids these particles from having the same quantum numbers. Therefore, for two electrons to occupy the same orbital, and thereby have the same orbital quantum number, they must have different spin quantum number. This also limits the number of electrons in the same orbital to two. The pairing of spins is often energetically favorable, and electron pairs therefore play a large role in chemistry. They can form a chemical bond between two atoms, or they can occur as a lone pair of valence electrons. They also fill the core levels of an atom. Because the spins are paired, the magnetic moment of the electrons cancel one another, and the pair's contributio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work ( pharmacology), and how to collec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
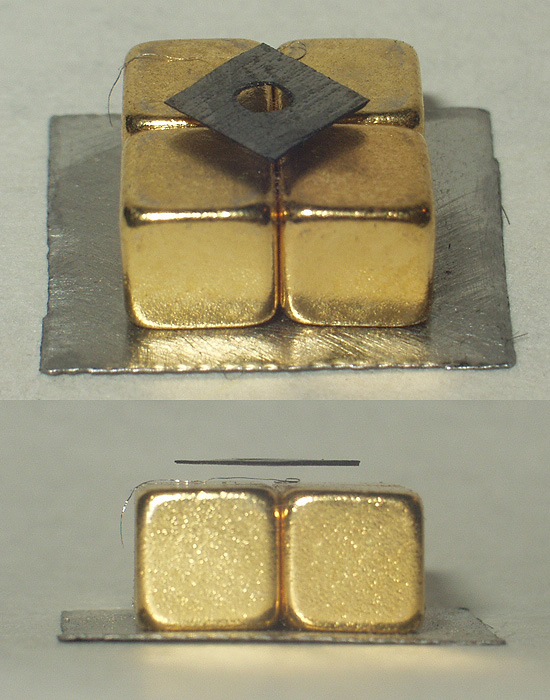
Diamagnetism
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The magnetic permeability of diamagnetic materials is less than the permeability of vacuum, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductor acts as a strong diamagnet because it repels a magnetic field entirely from its interior. Diamagnetism was first discovered when Anton Brugmans observed in 1778 that bismuth was r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
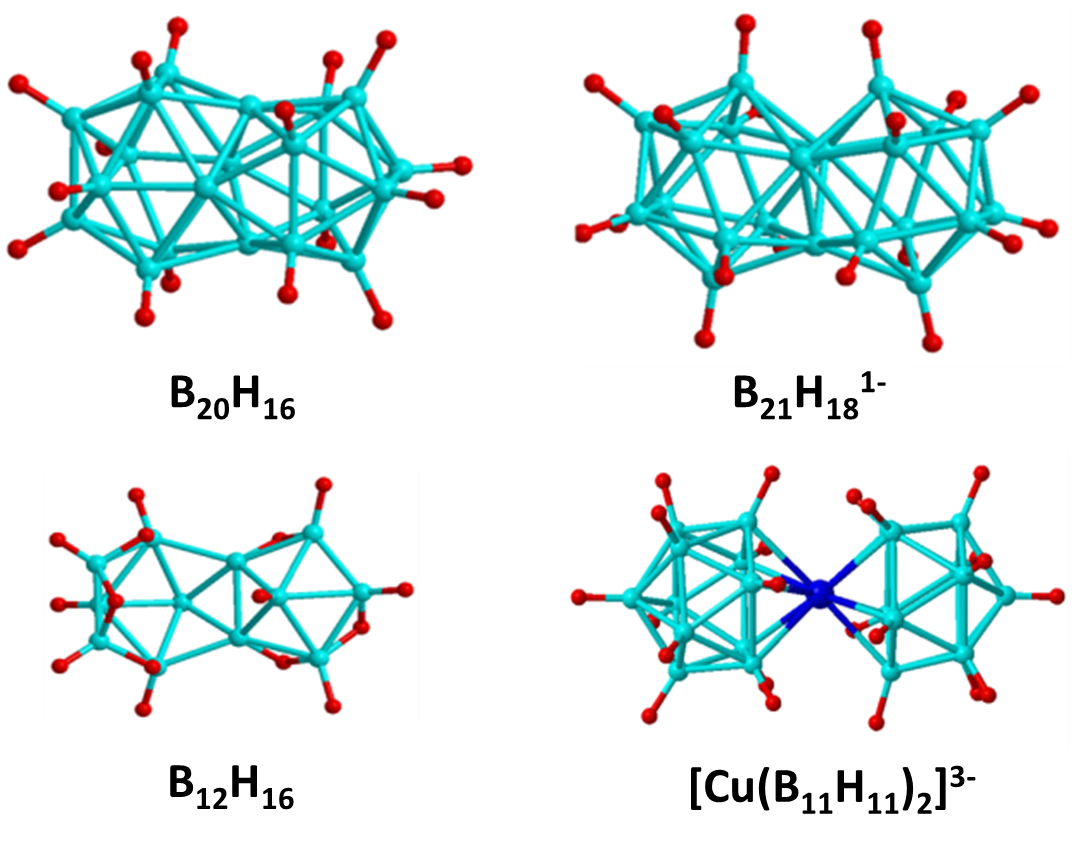
Jemmis Mno Rules
In chemistry, the Jemmis ''mno'' rules represent a unified rule for predicting and systematizing structures of compounds, usually clusters. The rules involve electron counting. They were formulated by Eluvathingal Devassy Jemmis to explain the structures of condensed polyhedral boranes such as , which are obtained by condensing polyhedral boranes by sharing a triangular face, an edge, a single vertex, or four vertices. These rules are additions and extensions to Wade's rules and polyhedral skeletal electron pair theory. The Jemmis ''mno'' rule provides the relationship between polyhedral boranes, condensed polyhedral boranes, and β-rhombohedral boron. This is similar to the relationship between benzene, condensed benzenoid aromatics, and graphite, shown by Hückel's 4''n'' + 2 rule, as well as the relationship between tetracoordinate tetrahedral carbon compounds and diamond. The Jemmis ''mno'' rules reduce to Hückel's rule when restricted to two dimensions and redu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frustrated Lewis Pair
A frustrated Lewis pair (FLP) is a compound or mixture containing a Lewis acid and a Lewis base that, because of steric hindrance, cannot combine to form a classical adduct. Many kinds of FLPs have been devised, and many simple substrates exhibit activation. The discovery that some FLPs split H2 triggered a rapid growth of research into FLPs. Because of their "unquenched" reactivity, such systems are reactive toward substrates that can undergo heterolysis. For example, many FLPs split hydrogen molecules. Thus, a mixture of tricyclohexylphosphine (PCy3) and tris(pentafluorophenyl)borane reacts with hydrogen to give the respective phosphonium and borate ions: :PCy3 + B(C6F5)3 + H2 -> PCy3 B(C6F5)3 This reactivity has been exploited to produce FLPs which catalyse hydrogenation reactions. Small molecule activation Frustrated Lewis pairs have been shown to activate many small molecules, either by inducing heterolysis or by coordination. Hydrogen The discovery that som ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
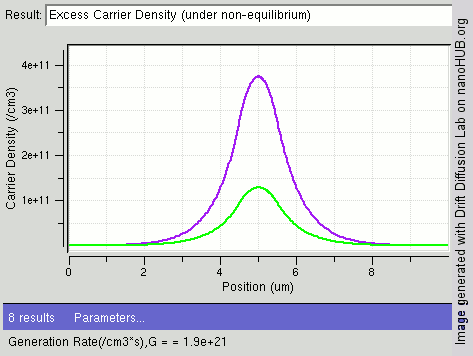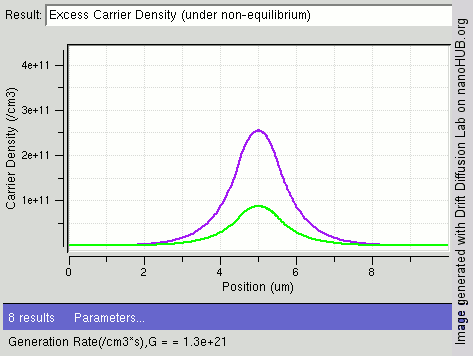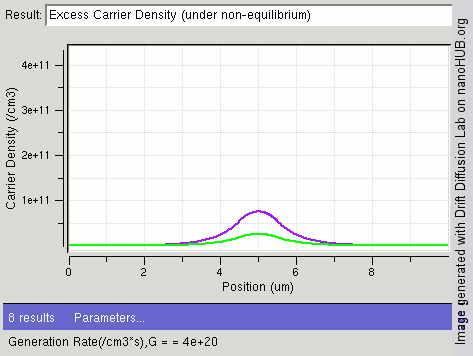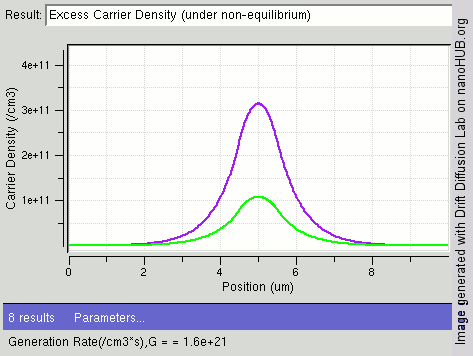
Carrier Generation And Recombination
In the solid-state physics of semiconductors, carrier generation and carrier recombination are processes by which mobile charge carriers (electrons and electron holes) are created and eliminated. Carrier generation and recombination processes are fundamental to the operation of many optoelectronic semiconductor devices, such as photodiodes, light-emitting diodes and laser diodes. They are also critical to a full analysis of p-n junction devices such as bipolar junction transistors and p-n junction diodes. The electron–hole pair is the fundamental unit of generation and recombination in inorganic semiconductors, corresponding to an electron transitioning between the valence band and the conduction band where generation of electron is a transition from the valence band to the conduction band and recombination leads to a reverse transition. Overview Like other solids, semiconductor materials have an electronic band structure determined by the crystal properties of the material ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vienna University Of Technology
TU Wien (TUW; german: Technische Universität Wien; still known in English as the Vienna University of Technology from 1975–2014) is one of the major universities in Vienna, Austria. The university finds high international and domestic recognition in teaching as well as in research, and it is a highly esteemed partner of innovation-oriented enterprises. It currently has about 28,100 students (29% women), eight faculties and about 5,000 staff members (3,800 academics). The university's teaching and research is focused on engineering, computer science, and natural sciences. History The institution was founded in 1815 by Emperor Francis I of Austria as the '' k.k. Polytechnische Institut'' (Imperial-Royal Polytechnic Institute). The first rector was Johann Joseph von Prechtl. It was renamed the ''Technische Hochschule'' (College of Technology) in 1872. When it began granting doctoral and higher degrees in 1975, it was renamed the ''Technische Universität Wien'' (Vienna Univers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phys
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which relates to the order of nature, or, in other words, to the regular succession of events." Physics is one of the most fundamental scientific disciplines, with its main goal being to understand how the universe behaves. "Physics is one of the most fundamental of the sciences. Scientists of all disciplines use the ideas of physics, including chemists who study the structure of molecules, paleontologists who try to reconstruct how dinosaurs walked, and climatologists who study how human activities affect the atmosphere and oceans. Physics is also the foundation of all engineering and technology. No engineer could design a flat-screen TV, an interplanetary spacecraft, or even a better mousetrap without first understanding the basic laws of physics. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin (physics)
Spin is a conserved quantity carried by elementary particles, and thus by composite particles (hadrons) and atomic nuclei. Spin is one of two types of angular momentum in quantum mechanics, the other being ''orbital angular momentum''. The orbital angular momentum operator is the quantum-mechanical counterpart to the classical angular momentum of orbital revolution and appears when there is periodic structure to its wavefunction as the angle varies. For photons, spin is the quantum-mechanical counterpart of the polarization of light; for electrons, the spin has no classical counterpart. The existence of electron spin angular momentum is inferred from experiments, such as the Stern–Gerlach experiment, in which silver atoms were observed to possess two possible discrete angular momenta despite having no orbital angular momentum. The existence of the electron spin can also be inferred theoretically from the spin–statistics theorem and from the Pauli exclusion principle—and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiferromagnetic
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism. The phenomenon of antiferromagnetism was first introduced by Lev Landau in 1933. Generally, antiferromagnetic order may exist at sufficiently low temperatures, but vanishes at and above the Néel temperature – named after Louis Néel, who had first identified this type of magnetic ordering. Above the Néel temperature, the material is typically paramagnetic. Measurement When no external field is applied, the antiferromagnetic structure corresponds to a vanishing total magnetization. In an external magnetic field, a kind of ferrimagnetic behavior may be displayed in the antiferromagnetic phase, with the absolute value of one of the sublattice magne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form ( native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cooper Pair
In condensed matter physics, a Cooper pair or BCS pair (Bardeen–Cooper–Schrieffer pair) is a pair of electrons (or other fermions) bound together at low temperatures in a certain manner first described in 1956 by American physicist Leon Cooper. Cooper pair Cooper showed that an arbitrarily small attraction between electrons in a metal can cause a paired state of electrons to have a lower energy than the Fermi energy, which implies that the pair is bound. In conventional superconductors, this attraction is due to the electron–phonon interaction. The Cooper pair state is responsible for superconductivity, as described in the BCS theory developed by John Bardeen, Leon Cooper, and John Schrieffer for which they shared the 1972 Nobel Prize. Although Cooper pairing is a quantum effect, the reason for the pairing can be seen from a simplified classical explanation. An electron in a metal normally behaves as a free particle. The electron is repelled from other electrons due ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |