|
Dimethylallyl Pyrophosphate
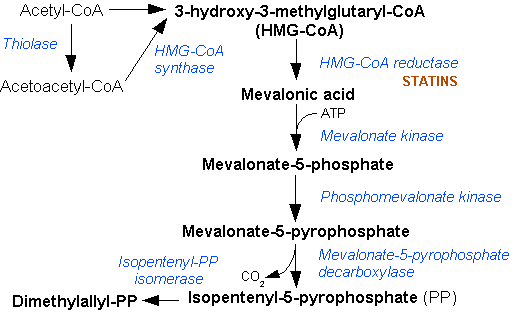
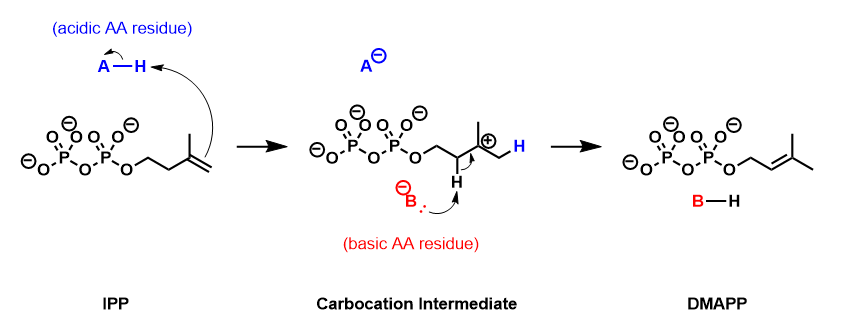
Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynthesis. It is an isomer of isopentenyl pyrophosphate (IPP) and exists in virtually all life forms. The enzyme isopentenyl pyrophosphate isomerase catalyzes isomerization between DMAPP and IPP. In the mevalonate pathway DMAPP is synthesised from mevalonic acid. In contrast, DMAPP is synthesised from HMBPP in the MEP pathway. At present, it is believed that there is crossover between the two pathways in organisms that use both pathways to create terpenes and terpenoid The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes" ...s, such as in plants, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mevalonate Pathway
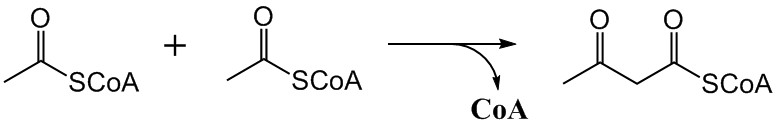
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP. It is best known as the target of statins, a class of cholesterol lowering drugs. Statins inhibit HMG-CoA reductase within the mevalonate pathway. Upper mevalonate pathway The mevalonate pathway of eukaryotes, archaea, and eubacteria all begin the same way. The sole carbon feed stock of the pathway is acetyl-CoA. The first step condenses two acetyl-CoA molecules to yield acetoacetyl-CoA. This is followed by a second condensation to form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylerythritol Phosphate
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-''C''-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP. Isoprenoid precursor biosynthesis The classical mevalonate pathway (MVA pathway or HMG-CoA reductase pathway) is a metabolic pathway for the biosynthesis of isoprenoid precursors: IPP and DMAPP. The MVA pathway is present in most eukaryotes and some bacteria. IPP and DMAPP serve as the basis for the biosynthesis of isoprenoid (terpenoid) molecules used in processes as diverse as protein prenylation, cell membrane maintenance, the synthesis of hormones, protein anchoring and ''N''-glycosylation in all three domains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopentenyl Pyrophosphate
Isopentenyl pyrophosphate (IPP, isopentenyl diphosphate, or IDP) is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway (commonly called the mevalonate pathway) and in the ''non-mevalonate'' MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids. Biosynthesis IPP is formed from acetyl-CoA via the mevalonate pathway (the "upstream" part), and then is isomerized to dimethylallyl pyrophosphate by the enzyme isopentenyl pyrophosphate isomerase. IPP can be synthesised via an alternative non-mevalonate pathway of isoprenoid precursor biosynthesis, the MEP pathway, where it is formed from (''E'')-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) by the enzyme HMB-PP reductase (LytB, IspH). The MEP pathway is present in many bacteria, apicomplexan protozoa such as malaria parasites, and in the plastids of higher pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopentenyl Pyrophosphate Isomerase
Isopentenyl pyrophosphate isomerase (, IPP isomerase), also known as Isopentenyl-diphosphate delta isomerase, is an isomerase that catalyzes the conversion of the relatively un-reactive isopentenyl pyrophosphate (IPP) to the more-reactive electrophile dimethylallyl pyrophosphate (DMAPP). This isomerization is a key step in the biosynthesis of isoprenoids through the mevalonate pathway and the MEP pathway. :isopentenyl diphosphate \rightleftharpoons dimethylallyl diphosphate This enzyme belongs to the family of isomerases, specifically those intramolecular oxidoreductases transposing C=C bonds. The systematic name of this enzyme class is isopentenyl-diphosphate Delta3-Delta2-isomerase. Other names in common use include isopentenylpyrophosphate Delta-isomerase, methylbutenylpyrophosphate isomerase, and isopentenylpyrophosphate isomerase. Enzyme mechanism IPP isomerase catalyzes the isomerization of IPP to DMAPP by an antarafacial transposition of hydrogen. The empirical e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mevalonic Acid
Mevalonic acid (MVA) is a key organic compound in biochemistry; the name is a contraction of dihydroxymethylvalerolactone. The carboxylate anion of mevalonic acid, which is the predominant form in biological environments, is known as ''mevalonate'' and is of major pharmaceutical importance. Drugs like statins (which lower levels of cholesterol) stop the production of mevalonate by inhibiting HMG-CoA reductase. Chemistry Mevalonic acid is very soluble in water and polar organic solvents. It exists in equilibrium with its lactone form, called mevalonolactone, that is formed by internal condensation of its terminal alcohol and carboxylic acid functional groups. Biology Mevalonic acid is a precursor in the biosynthetic pathway known as the mevalonate pathway that produces terpenes and steroids. Mevalonic acid is the primary precursor of isopentenyl pyrophosphate (IPP), that is in turn the basis for all terpenoids. Mevalonic acid is chiral and the (3''R'')-enantiomer In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(E)-4-Hydroxy-3-methyl-but-2-enyl Pyrophosphate
(''E'')-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP or HMB-PP) is an intermediate of the MEP pathway (non-mevalonate pathway) of isoprenoid biosynthesis. The enzyme HMB-PP synthase (GcpE, IspG) catalyzes the conversion of 2-''C''-methyl-D-erythritol 2,4-cyclodiphosphate (MEcPP) into HMB-PP. HMB-PP is then converted further to isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) by HMB-PP reductase (LytB, IspH). HMB-PP is an essential metabolite in most pathogenic bacteria including ''Mycobacterium tuberculosis'' as well as in malaria Malaria is a mosquito-borne infectious disease that affects humans and other animals. Malaria causes symptoms that typically include fever, tiredness, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. ... parasites, but is absent from the human host. HMB-PP is the physiological activator ("phosphoantigen") for human Vg9/Vd2 T cells, Vγ9/Vδ2 T cells, the major gamma/delta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-mevalonate Pathway
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-''C''-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP. Isoprenoid precursor biosynthesis The classical mevalonate pathway (MVA pathway or HMG-CoA reductase pathway) is a metabolic pathway for the biosynthesis of isoprenoid precursors: IPP and DMAPP. The MVA pathway is present in most eukaryotes and some bacteria. IPP and DMAPP serve as the basis for the biosynthesis of isoprenoid (terpenoid) molecules used in processes as diverse as protein prenylation, cell membrane maintenance, the synthesis of hormones, protein anchoring and ''N''-glycosylation in all three domai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene, is a major component of the common solvent, turpentine. History and terminology The term ''terpene'' was coined in 1866 by the German chemist August Kekulé to denote all hydrocarbons having the empirical formula C10H16, of which camphene was one. Previously, many hydrocarbons having the empirical formula C10H16 had been called "camphene", but many other hydrocarbons of the same composition had had different names. Kekulé coined the term "terpene" in order to reduce the confusion. The name "terpene" is a shortened form of "terpentine", an obsolete spelling of "turpentine". Although sometim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpenoid
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually containing oxygen. When combined with the hydrocarbon terpenes, terpenoids comprise about 80,000 compounds. They are the largest class of plant secondary metabolites, representing about 60% of known natural products. Many terpenoids have substantial pharmacological bioactivity and are therefore of interest to medicinal chemists. Plant terpenoids are used for their aromatic qualities and play a role in traditional herbal remedies. Terpenoids contribute to the scent of eucalyptus, the flavors of cinnamon, cloves, and ginger, the yellow color in sunflowers, and the red color in tomatoes. Well-known terpenoids include citral, menthol, camphor, salvinorin A in the plant ''Salvia div ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mevalonate Pathway
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP. It is best known as the target of statins, a class of cholesterol lowering drugs. Statins inhibit HMG-CoA reductase within the mevalonate pathway. Upper mevalonate pathway The mevalonate pathway of eukaryotes, archaea, and eubacteria all begin the same way. The sole carbon feed stock of the pathway is acetyl-CoA. The first step condenses two acetyl-CoA molecules to yield acetoacetyl-CoA. This is followed by a second condensation to form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterol Synthesis
Sterol is an organic compound with formula , whose molecule is derived from that of gonane by replacement of a hydrogen atom in position 3 by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the gonane structure, additional functional groups, and/or modified ring systems derived from gonane are called steroids. Therefore, sterols are a subgroup of the steroids. They occur naturally in most eukaryotes, including plants, animals, and fungi, and can also be produced by some bacteria (however likely with different functions). The most familiar type of animal sterol is cholesterol, which is vital to cell membrane structure, and functions as a precursor to fat-soluble vitamins and steroid hormones. While technically alcohols, sterols are classified by biochemists as lipids (fats in the broader sense of the term). Types Sterols of plants are called ''phytosterols'' and sterols of animals are called ''zoosterols''. The most importan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphates
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Like most functional groups, organophosphates occur in a diverse range of forms, with important examples including key biomolecules such as DNA, RNA and ATP, as well as many insecticides, herbicides, nerve agents and flame retardants. OPEs have been widely used in various products as flame retardants, plasticizers, and performance additives to engine oil. The popularity of OPEs as flame retardants came as a substitution for the highly regulated brominated flame retardants. The low cost of production and compatibility to diverse polymers made OPEs to be widely used in industry including textile, furniture, electronics as plasticizers and flame retardants. These compounds are added to the final product p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |