|
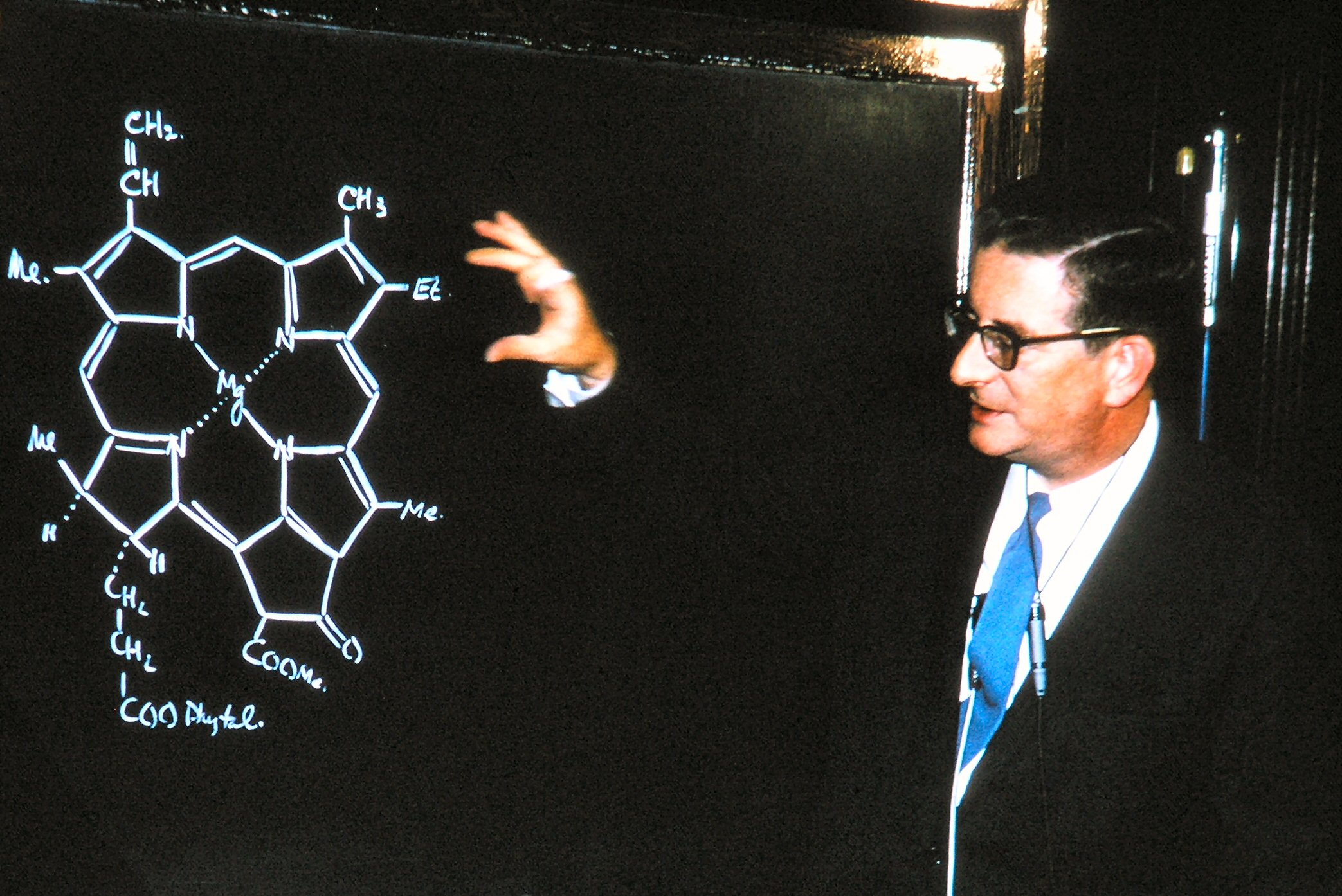
Chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to absorb energy from light. Chlorophylls absorb light most strongly in the blue portion of the electromagnetic spectrum as well as the red portion. Conversely, it is a poor absorber of green and near-green portions of the spectrum. Hence chlorophyll-containing tissues appear green because green light, diffusively reflected by structures like cell walls, is less absorbed. Two types of chlorophyll exist in the photosystems of green plants: chlorophyll ''a'' and ''b''. History Chlorophyll was first isolated and named by Joseph Bienaimé Caventou and Pierre Joseph Pelletier in 1817. The presence of magnesium in chlorophyll was discovered in 1906, and was that element's first detection in living tissue. After initial work done by German chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll Ab Spectra-en
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to absorb energy from light. Chlorophylls absorb light most strongly in the blue portion of the electromagnetic spectrum as well as the red portion. Conversely, it is a poor absorber of green and near-green portions of the spectrum. Hence chlorophyll-containing tissues appear green because green light, diffusively reflected by structures like cell walls, is less absorbed. Two types of chlorophyll exist in the photosystems of green plants: chlorophyll ''a'' and ''b''. History Chlorophyll was first isolated and named by Joseph Bienaimé Caventou and Pierre Joseph Pelletier in 1817. The presence of magnesium in chlorophyll was discovered in 1906, and was that element's first detection in living tissue. After initial work done by German chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroplast
A chloroplast () is a type of membrane-bound organelle known as a plastid that conducts photosynthesis mostly in plant and algal cells. The photosynthetic pigment chlorophyll captures the energy from sunlight, converts it, and stores it in the energy-storage molecules ATP and NADPH while freeing oxygen from water in the cells. The ATP and NADPH is then used to make organic molecules from carbon dioxide in a process known as the Calvin cycle. Chloroplasts carry out a number of other functions, including fatty acid synthesis, amino acid synthesis, and the immune response in plants. The number of chloroplasts per cell varies from one, in unicellular algae, up to 100 in plants like ''Arabidopsis'' and wheat. A chloroplast is characterized by its two membranes and a high concentration of chlorophyll. Other plastid types, such as the leucoplast and the chromoplast, contain little chlorophyll and do not carry out photosynthesis. Chloroplasts are highly dynamic—they circulat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll B
} Chlorophyll ''b'' is a form of chlorophyll. Chlorophyll ''b'' helps in photosynthesis by absorbing light energy. It is more soluble than chlorophyll ''a'' in polar solvents because of its carbonyl group. Its color is green, and it primarily absorbs blue light. In land plants, the light-harvesting antennae around photosystem II contain the majority of chlorophyll ''b''. Hence, in shade-adapted chloroplasts, which have an increased ratio of photosystem II to photosystem I, there is a higher ratio of chlorophyll ''b'' to chlorophyll ''a''. This is adaptive, as increasing chlorophyll ''b'' increases the range of wavelengths absorbed by the shade chloroplasts. Biosynthesis The Chlorophyll ''b'' biosynthetic pathway utilizes a variety of enzymes. In most plants, chlorophyll is derived from glutamate and is synthesised along a branched pathway that is shared with heme and siroheme. The initial steps incorporate glutamic acid into 5-aminolevulinic acid (ALA); two molecules of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll F
Chlorophyll ''f'' is a type form of chlorophyll that absorbs further in the red (infrared light) than other chlorophylls. In 2010, it was reported from stromatolites from Western Australia's Shark Bay. The finding was made by scientists at the University of Sydney led by Professor Min Chen, and is the first discovery of a new form of chlorophyll in 60 years. However, the function of chlorophyll ''f'' in photosynthetic reactions is uncertain and the ecological distribution of chlorophyll ''f'' remains unknown. Chlorophyll ''f'' has been shown to support some of the roles in photosynthetic reactions, in both the energy transfer and in the charge separation processes. Based on NMR data, optical and mass spectra and density functional theory Density-functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plant
Plants are predominantly photosynthetic eukaryotes of the kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all current definitions of Plantae exclude the fungi and some algae, as well as the prokaryotes (the archaea and bacteria). By one definition, plants form the clade Viridiplantae (Latin name for "green plants") which is sister of the Glaucophyta, and consists of the green algae and Embryophyta (land plants). The latter includes the flowering plants, conifers and other gymnosperms, ferns and their allies, hornworts, liverworts, and mosses. Most plants are multicellular organisms. Green plants obtain most of their energy from sunlight via photosynthesis by primary chloroplasts that are derived from endosymbiosis with cyanobacteria. Their chloroplasts contain chlorophylls a and b, which gives them their green color. Some plants are parasitic or mycotrophic and have lost the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorin
In organic chemistry, chlorins are tetrapyrrole pigments that are partially hydrogenated porphyrins. The parent chlorin is an unstable compound which undergoes air oxidation to porphine. The name chlorin derives from chlorophyll. Chlorophylls are magnesium-containing chlorins and occur as photosynthetic pigments in chloroplasts. The reduced chlorin variants are present in bacteriochlorophylls and are named ‘bacteriochlorins’ and ‘isobacteriochlorins’. Chlorins are excellent photosensitizing agents. Various synthetic chlorins analogues such as m-tetrahydroxyphenylchlorin (mTHPC) and mono-L-aspartyl chlorin e6 are effectively employed in experimental photodynamic therapy as photosensitizer. Chlorophylls The most abundant chlorin is the photosynthetic pigment chlorophyll. Chlorophylls have a fifth, ketone-containing ring unlike the chlorins. Diverse chlorophylls exists, such as chlorophyll ''a'', chlorophyll ''b'', chlorophyll ''d'', chlorophyll ''e'', chlorophyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alga
Algae (; singular alga ) is an informal term for a large and diverse group of photosynthetic eukaryotic organisms. It is a polyphyletic grouping that includes species from multiple distinct clades. Included organisms range from unicellular microalgae, such as ''Chlorella,'' ''Prototheca'' and the diatoms, to multicellular forms, such as the giant kelp, a large brown alga which may grow up to in length. Most are aquatic and autotrophic (they generate food internally) and lack many of the distinct cell and tissue types, such as stomata, xylem and phloem that are found in land plants. The largest and most complex marine algae are called seaweeds, while the most complex freshwater forms are the ''Charophyta'', a division of green algae which includes, for example, ''Spirogyra'' and stoneworts. No definition of algae is generally accepted. One definition is that algae "have chlorophyll ''a'' as their primary photosynthetic pigment and lack a sterile covering of cells around t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Richard Willstätter
Richard Martin Willstätter FRS(For) HFRSE (, 13 August 1872 – 3 August 1942) was a German organic chemist whose study of the structure of plant pigments, chlorophyll included, won him the 1915 Nobel Prize for Chemistry. Willstätter invented paper chromatography independently of Mikhail Tsvet. Life Willstätter was born into a Jewish family in Karlsruhe. He was the son of Maxwell (Max) Willstätter, a textile merchant, and his wife, Sophie Ulmann. He went to school at the Karlsruhe Gymnasium and, when his family moved to Nuremberg, he attended the Technical School there. At age 18 he entered the University of Munich to study science and stayed for the next fifteen years. He was in the Department of Chemistry, first as a student of Alfred Einhorn—he received his doctorate in 1894 – then as a faculty member. His doctoral thesis was on the structure of cocaine. Willstätter continued his research into other alkaloids and synthesized several of them. In 1896 he was named Lec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert Burns Woodward
Robert Burns Woodward (April 10, 1917 – July 8, 1979) was an American organic chemist. He is considered by many to be the most preeminent synthetic organic chemist of the twentieth century, having made many key contributions to the subject, especially in the synthesis of complex natural products and the determination of their molecular structure. He also worked closely with Roald Hoffmann on theoretical studies of chemical reactions. He was awarded the Nobel Prize in Chemistry in 1965. Early life and education Woodward was born in Boston, Massachusetts, on April 10, 1917. He was the son of Margaret Burns (an immigrant from Scotland who claimed to be a descendant of the poet, Robert Burns) and her husband, Arthur Chester Woodward, himself the son of Roxbury apothecary, Harlow Elliot Woodward. His father was one of the many victims of the 1918 influenza pandemic of 1918. From a very early age, Woodward was attracted to and engaged in private study of chemistry while he att ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanobacteria
Cyanobacteria (), also known as Cyanophyta, are a phylum of gram-negative bacteria that obtain energy via photosynthesis. The name ''cyanobacteria'' refers to their color (), which similarly forms the basis of cyanobacteria's common name, blue-green algae, although they are not usually scientifically classified as algae. They appear to have originated in a freshwater or terrestrial environment. Sericytochromatia, the proposed name of the paraphyletic and most basal group, is the ancestor of both the non-photosynthetic group Melainabacteria and the photosynthetic cyanobacteria, also called Oxyphotobacteria. Cyanobacteria use photosynthetic pigments, such as carotenoids, phycobilins, and various forms of chlorophyll, which absorb energy from light. Unlike heterotrophic prokaryotes, cyanobacteria have internal membranes. These are flattened sacs called thylakoids where photosynthesis is performed. Phototrophic eukaryotes such as green plants perform photosynthesis in plast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hans Fischer
Hans Fischer (; 27 July 1881 – 31 March 1945) was a German organic chemist and the recipient of the 1930 Nobel Prize for Chemistry "for his researches into the constitution of haemin and chlorophyll and especially for his synthesis of haemin." Biography Early years Fischer was born on July 27,1881 in Höchst on river Main, now a city district of Frankfurt located in Germany. His parents were Dr. Eugen Fischer, Director of the firm of Kalle & Co, Wiesbaden, and Privatdozent at the Technical High School, Stuttgart, and Anna Herdegen was his mother. He went to a primary school in Stuttgart, and later to the "Humanistisches Gymnasium" in Wiesbaden, matriculating in 1899. He read chemistry and medicine, first at the University of Lausanne and then at Marburg. He graduated in 1904 obtaining his chemistry degree, 2 years later in 1906 he licensed for medicine and in 1908 he qualified for his M.D. including the Nobel Lecture, December 11, 1930 ''On Haemin and the Relationships b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |