|
Biocompatibility
Biocompatibility is related to the behavior of biomaterials in various contexts. The term refers to the ability of a material to perform with an appropriate host response in a specific situation. The ambiguity of the term reflects the ongoing development of insights into how biomaterials interact with the human body and eventually how those interactions determine the clinical success of a medical device (such as pacemaker, hip replacement or stent). Modern medical devices and prostheses are often made of more than one material so it might not always be sufficient to talk about the biocompatibility of a specific material. Since the immune response and repair functions in the body are so complicated it is not adequate to describe the biocompatibility of a single material in relation to a single cell type or tissue. Sometimes one hears of biocompatibility testing that is a large battery of in vitro test that is used in accordance with ISO 10993 (or other similar standards) to det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomaterials
A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose, either a therapeutic (treat, augment, repair, or replace a tissue function of the body) or a diagnostic one. As a science, biomaterials is about fifty years old. The study of biomaterials is called biomaterials science or biomaterials engineering. It has experienced steady and strong growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science. Note that a biomaterial is different from a biological material, such as bone, that is produced by a biological system. Additionally, care should be exercised in defining a biomaterial as biocompatible, since it is application-specific. A biomaterial that is biocompatible or suitable for one application may not be biocompatible in another. Introduction Bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biocompatible Material
A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose, either a therapeutic (treat, augment, repair, or replace a tissue function of the body) or a diagnostic one. As a science, biomaterials is about fifty years old. The study of biomaterials is called biomaterials science or biomaterials engineering. It has experienced steady and strong growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science. Note that a biomaterial is different from a biological material, such as bone, that is produced by a biological system. Additionally, care should be exercised in defining a biomaterial as biocompatible, since it is application-specific. A biomaterial that is biocompatible or suitable for one application may not be biocompatible in another. Introduction Bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomaterial
A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose, either a therapeutic (treat, augment, repair, or replace a tissue function of the body) or a diagnostic one. As a science, biomaterials is about fifty years old. The study of biomaterials is called biomaterials science or biomaterials engineering. It has experienced steady and strong growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science. Note that a biomaterial is different from a biological material, such as bone, that is produced by a biological system. Additionally, care should be exercised in defining a biomaterial as biocompatible, since it is application-specific. A biomaterial that is biocompatible or suitable for one application may not be biocompatible in another. Introduction Bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomaterials
A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose, either a therapeutic (treat, augment, repair, or replace a tissue function of the body) or a diagnostic one. As a science, biomaterials is about fifty years old. The study of biomaterials is called biomaterials science or biomaterials engineering. It has experienced steady and strong growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science. Note that a biomaterial is different from a biological material, such as bone, that is produced by a biological system. Additionally, care should be exercised in defining a biomaterial as biocompatible, since it is application-specific. A biomaterial that is biocompatible or suitable for one application may not be biocompatible in another. Introduction Bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
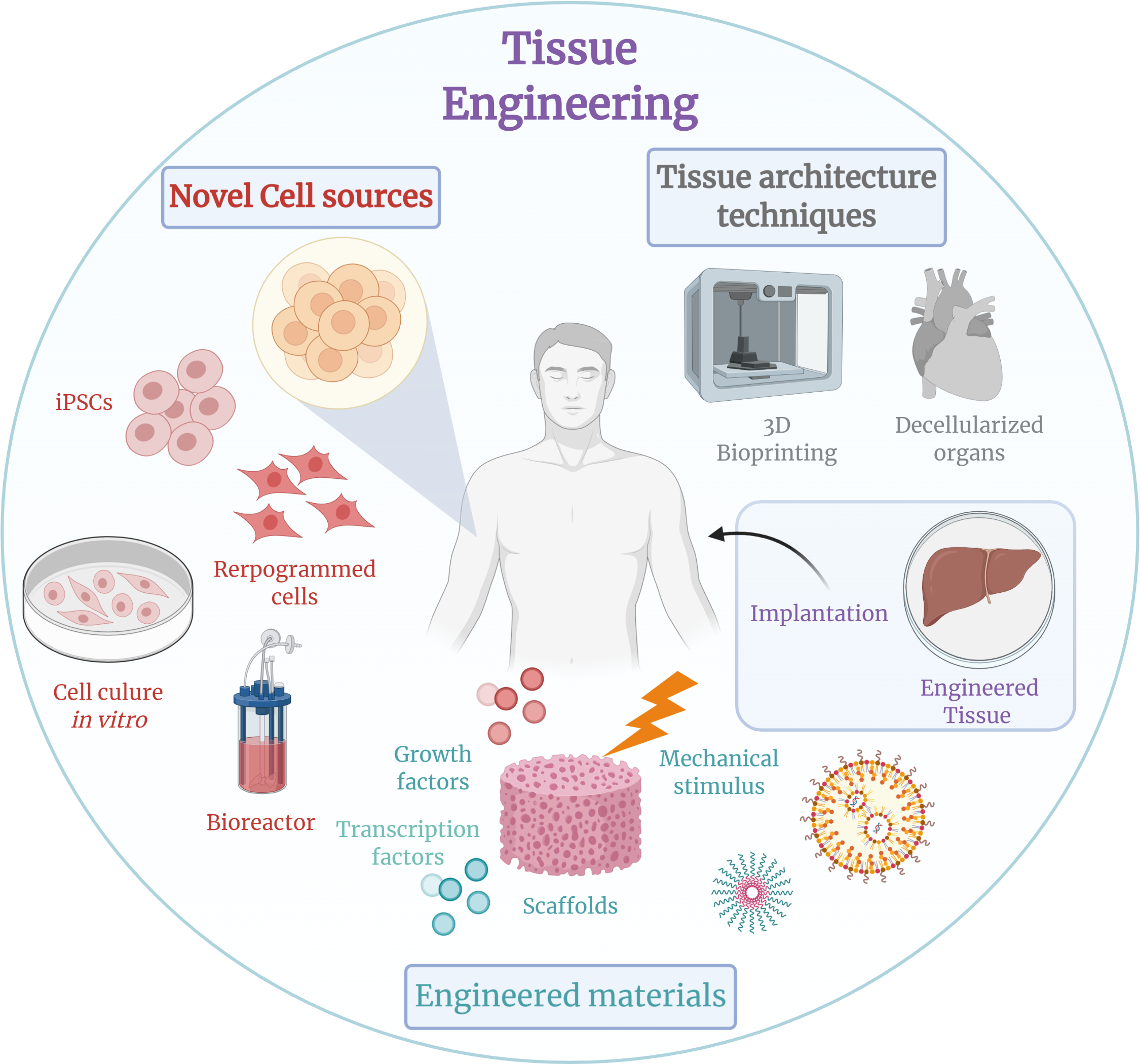
Tissue Engineering
Tissue engineering is a biomedical engineering discipline that uses a combination of Cell (biology), cells, engineering, Materials science, materials methods, and suitable biochemistry, biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biology, biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field of its own. While most definitions of tissue engineering cover a broad range of applications, in practice the term is closely associated with applications that repair or replace portions of or whole tissues (i.e. bone, Autologous chondrocyte implantation, cartilage, blood vessels, Urinary bladder, bladder, skin, muscle etc.). Often, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Biocompatibility
Titanium was first introduced into surgeries in the 1950s after having been used in dentistry for a decade prior. It is now the metal of choice for prosthetics, internal fixation, inner body devices, and instrumentation. Titanium is used from head to toe in biomedical implants. One can find titanium in neurosurgery, bone conduction hearing aids, false eye implants, spinal fusion cages, pacemakers, toe implants, and shoulder/elbow/hip/knee replacements along with many more. The main reason why titanium is often used in the body is due to titanium's biocompatibility and, with surface modifications, bioactive surface. The surface characteristics that affect biocompatibility are surface texture, steric hindrance, binding sites, and hydrophobicity (wetting). These characteristics are optimized to create an ideal cellular response. Some medical implants, as well as parts of surgical instruments are coated with titanium nitride (TiN). Biocompatibility Titanium is considered the most bioco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ISO 10993
The ISO 10993 set entails a series of standards for evaluating the biocompatibility of medical devices to manage biological risk. These documents were preceded by the Tripartite agreement and is a part of the international harmonisation of the safe use evaluation of medical devices. For the purpose of the ISO 10993 family of standards, ''biocompatibility'' is defined as the "ability of a medical device or material to perform with an appropriate host response in a specific application". ISO 10993-1:2009 & FDA endpoints for consideration The following table provides a framework for the development of a biocompatibility evaluation. Different biological endpoints may require evaluation for particular medical devices, including either additional or fewer endpoints than indicated. If it is unclear in which category a device falls, consulting device-specific guidances or contacting the appropriate US Food and Drug Administration (FDA) review division for more information is possible. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medical Grade Silicone
Medical grade silicones are silicones tested for biocompatibility and are appropriate to be used for medical applications. In the United States, the Food and Drug Administration (FDA) Center for Devices and Radiological Health (CDRH) regulates devices implanted into the body. It does not regulate materials other than certain dental materials. The FDA regulate silicones used in food contact under the auspices of the Center for Food Safety and Nutrition (CFSAN) and for use in pharmaceuticals under the auspices of the Center for Drug Evaluation and Research (CDER). Medical grade silicones are generally grouped into three categories: non implantable, short term implantable, and long-term implantable. Materials approved as Class V and VI can be considered medical grade. Most medical grade silicones are at least Class VI certified. Silicone suppliers and some silicone prototyping companies provide guidelines for material use. Uses *Tubing * Drains *Feeding tubes *Catheters *Implants ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hip Replacement
Hip replacement is a surgical procedure in which the hip joint is replaced by a prosthetic implant, that is, a hip prosthesis. Hip replacement surgery can be performed as a total replacement or a hemi (half) replacement. Such joint replacement orthopaedic surgery is generally conducted to relieve arthritis pain or in some hip fractures. A total hip replacement (total hip arthroplasty or THA) consists of replacing both the acetabulum and the femoral head while hemiarthroplasty generally only replaces the femoral head. Hip replacement is one of the most common orthopaedic operations, though patient satisfaction varies widely. Approximately 58% of total hip replacements are estimated to last 25 years. The average cost of a total hip replacement in 2012 was $40,364 in the United States, and about $7,700 to $12,000 in most European countries. Medical uses Total hip replacement is most commonly used to treat joint failure caused by osteoarthritis. Other indications include rheumatoi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dorland's Medical Dictionary
''Dorland's'' is the brand name of a family of medical reference works (including dictionaries, spellers and word books, and spell-check software) in various media spanning printed books, CD-ROMs, and online content. The flagship products are ''Dorland's Illustrated Medical Dictionary'' (currently in its 33rd edition) and ''Dorland's Pocket Medical Dictionary'' (currently in its 30th edition). The principal dictionary was first published in 1890 as the ''American Illustrated Medical Dictionary'', including 770 pages. The pocket edition, called the ''American Pocket Medical Dictionary'', was first published in 1898, consisting of just over 500 pages. With the death of the editor William Alexander Newman Dorland, AM, MD in 1956, the dictionaries were retitled to incorporate his name, which was how they had generally come to be known. The illustrated dictionary had grown to 2144 pages for the 33rd edition. The dictionaries were historically published by Saunders. List of products ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medical Device
A medical device is any device intended to be used for medical purposes. Significant potential for hazards are inherent when using a device for medical purposes and thus medical devices must be proved safe and effective with reasonable assurance before regulating governments allow marketing of the device in their country. As a general rule, as the associated risk of the device increases the amount of testing required to establish safety and efficacy also increases. Further, as associated risk increases the potential benefit to the patient must also increase. Discovery of what would be considered a medical device by modern standards dates as far back as c. 7000 BC in Baluchistan where Neolithic dentists used flint-tipped drills and bowstrings. Study of archeology and Roman medical literature also indicate that many types of medical devices were in widespread use during the time of ancient Rome. In the United States it wasn't until the Federal Food, Drug, and Cosmetic Act (F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |