|
Avidin
Avidin is a tetrameric biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs. Dimeric members of the avidin family are also found in some bacteria. In chicken egg white, avidin makes up approximately 0.05% of total protein (approximately 1800 μg per egg). The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of the avidin-biotin complex is measured to be ''K''D ≈ 10−15 M, making it one of the strongest known non-covalent bonds. In its tetrameric form, avidin is estimated to be 66–69 k Da in size. 10% of the molecular weight is contributed by carbohydrate, composed of four to five mannose and three N-acetylglucosamine residues The carbohydrate moieties of avidin contain at least three unique oligosaccharide structural types that are similar in structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptavidin
Streptavidin is a 66.0 (tetramer) kDa protein purified from the bacterium '' Streptomyces avidinii''. Streptavidin homo-tetramers have an extraordinarily high affinity for biotin (also known as vitamin B7 or vitamin H). With a dissociation constant (Kd) on the order of ≈10−14 mol/L, the binding of biotin to streptavidin is one of the strongest non-covalent interactions known in nature. Streptavidin is used extensively in molecular biology and bionanotechnology due to the streptavidin-biotin complex's resistance to organic solvents, denaturants (e.g. guanidinium chloride), detergents (e.g. SDS, Triton X-100), proteolytic enzymes, and extremes of temperature and pH. Structure The crystal structure of streptavidin with biotin bound was reported by two groups in 1989. The structure was solved using multi wavelength anomalous diffraction by Hendrickson et al. at Columbia University and using multiple isomorphous replacement by Weber et al. at E. I. DuPont Central Resea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biotin Structure
Biotin (or vitamin B7) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name ''biotin'', borrowed from the German , derives from the Ancient Greek word (; 'life') and the suffix "-in" (a suffix used in chemistry usually to indicate 'forming'). Chemical description Biotin is classified as a heterocyclic compound, with a sulfur-containing ring fused ureido and tetrahydrothiophene group. A C5-carboxylic acid side chain is appended to one of the rings. The ureido ring, containing the −N−CO−N− group, serves as the carbon dioxide carrier in carboxylation reactions. Biotin is a coenzyme for five carboxylase enzymes, which are involved in the catabolism of amino acids and fatty acids, synthesis of fatty acids, and gluconeogenesis. Biotinylation of histone proteins in nuclear chromatin plays a role in chromatin stability and ge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biotin
Biotin (or vitamin B7) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name ''biotin'', borrowed from the German , derives from the Ancient Greek word (; 'life') and the suffix "-in" (a suffix used in chemistry usually to indicate 'forming'). Chemical description Biotin is classified as a heterocyclic compound, with a sulfur-containing ring fused ureido and tetrahydrothiophene group. A C5-carboxylic acid side chain is appended to one of the rings. The ureido ring, containing the −N−CO−N− group, serves as the carbon dioxide carrier in carboxylation reactions. Biotin is a coenzyme for five carboxylase enzymes, which are involved in the catabolism of amino acids and fatty acids, synthesis of fatty acids, and gluconeogenesis. Biotinylation of histone proteins in nuclear chromatin plays a role in chromatin stability an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilchek
Meir Wilchek (Hebrew: מאיר אשר וילצ'ק, born 17 October 1935) is an Israeli biochemist. He is a professor at the Weizmann Institute of Science. Early life and education Meir Wilchek was born in Warsaw, Poland, scion of a rabbinical family. During the Holocaust, he escaped from the German-occupied territories to the territories occupied by Russia, and was transferred to Siberia, while his father, who served as a community rabbi in Warsaw, was killed in Flossenbürg concentration camp. He survived, and immigrated to Israel in 1949 with his mother and sister. He graduated with B.Sc. in chemistry from Bar Ilan university and Ph.D. in biochemistry from the Weizmann Institute of Science. Wilchek has published over 400 scientific papers, and consulted various biotech companies. He was also in the party list of Mafdal and Meimad for the Knesset. Scientific contributions Meir Wilchek is known for his research in the field of biorecognition or affinity phenomenon, and its variou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ELISPOT
The enzyme-linked immune absorbent spot (ELISpot) is a type of assay that focuses on quantitatively measuring the frequency of cytokine secretion for a single cell. The ELISpot Assay is also a form of immunostaining since it is classified as a technique that uses antibodies to detect a protein analyte, with the word analyte referring to any biological or chemical substance being identified or measured. The FluoroSpot Assay is a variation of the ELISpot assay. The FluoroSpot Assay uses fluorescence in order to analyze multiple analytes, meaning it can detect the secretion of more than one type of protein. History Cecil Czerkinsky first described ELISpot in 1983 as a new way to quantify the production of an antigen-specific immunoglobulin by hybridoma cells. In 1988, Czerkinsky developed an ELISA spot assay that quantified the secretion of a lymphokine by T cells. In the same year, dual-color ELISpot was combined with computer imaging for the first time, which allowed for the enu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrameric Protein
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits (such as glutathione S-transferase), and heterotetramers are complexes of different subunits. A tetramer can be assembled as dimer of dimers with two homodimer subunits (such as sorbitol dehydrogenase), or two heterodimer subunits (such as hemoglobin). Subunit interactions in tetramers The interactions between subunits forming a tetramer is primarily determined by non covalent interaction. Hydrophobic effects, hydrogen bonds and electrostatic interactions are the primary sources for this binding process between subunits. For homotetrameric proteins such as Sorbitol dehydrogenase (SDH), the structure is believed to have evolved going from a monomeric to a dimeric and finally a tetrameric structure in evolution. The binding process in SDH and many other tetrameric enzymes can be described by the gain in free energy which can be determined from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation Constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction: : A_\mathit B_\mathit \mathit A + \mathit B in which a complex \ce_x \ce_y breaks down into ''x'' A subunits and ''y'' B subunits, the dissociation constant is defined as : K_D = \frac where and ''x'' B''y''are the equilibrium concentrations of A, B, and the complex A''x'' B''y'', respectively. One reason for the popularity of the dissociation constant in biochemistry and pharmacology is that in the frequently enc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esmond Emerson Snell
Esmond Emerson Snell (September 22, 1914 – December 9, 2003) was an American biochemist who spent his career researching vitamins and nutritional requirements of bacteria and yeast. He is well known for his study of lactic acid-producing bacteria, developing microbiological assays for a number of key nutrients; the discovery of more than half of known vitamins has been attributed to the use of this work. He discovered several B vitamins, including folic acid, and characterized the biochemistry of vitamin B6 (also known as pyrixodal). Early life and education The fourth of five children, Snell was born in 1914 in Salt Lake City, Utah to parents who met while serving as Mormon missionaries. The family moved several times in Wyoming and Utah before settling in Provo, Utah so that the children could attend Brigham Young University. Snell became interested in chemistry during high school and went on to study chemistry at BYU; he also – "reluctantly", as he remembered later – s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Egg White
Egg white is the clear liquid (also called the albumen or the glair/glaire) contained within an egg. In chickens it is formed from the layers of secretions of the anterior section of the hen's oviduct during the passage of the egg. It forms around fertilized or unfertilized egg yolks. The primary natural purpose of egg white is to protect the yolk and provide additional nutrition for the growth of the embryo (when fertilized). Egg white consists primarily of about 90% water into which about 10% proteins (including albumins, mucoproteins, and globulins) are dissolved. Unlike the yolk, which is high in lipids (fats), egg white contains almost no fat, and carbohydrate content is less than 1%. Egg whites contain about 56% of the protein in the egg. Egg white has many uses in food (e.g. meringue, mousse) as well as many other uses (e.g. in the preparation of vaccines such as those for influenza). Composition Egg white makes up around two-thirds of a chicken egg by weight. Wate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
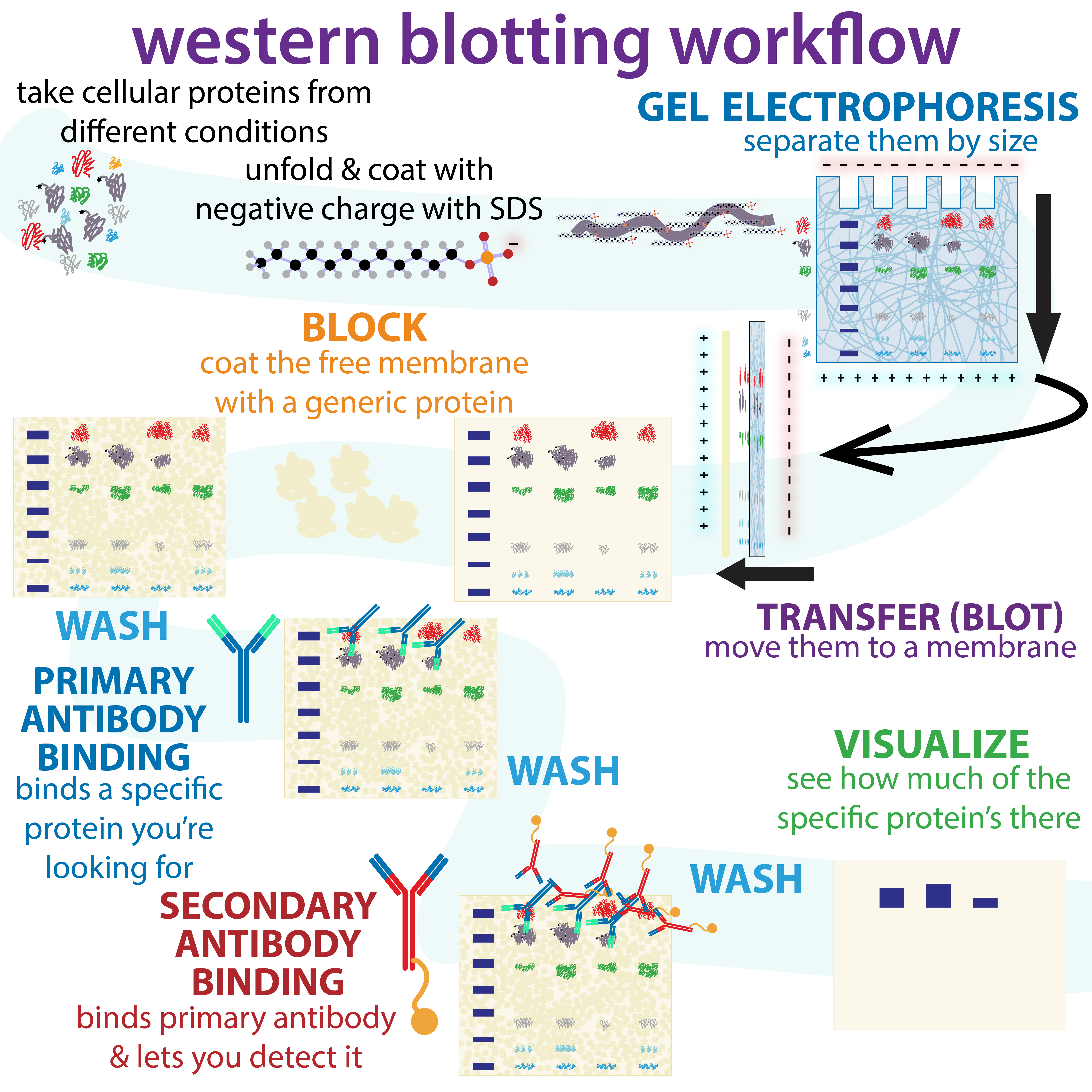
Western Blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detecting the proteins, this technique is also utilized to visualize, distinguish, and quantify the different proteins in a complicated protein combination. Western blot technique uses three elements to achieve its task of separating a specific protein from a complex: separation by size, transfer of protein to a solid support, and marking target protein using a primary and secondary antibody to visualize. A synthetic or animal-derived antibody (known as the primary antibody) is created that recognizes and binds to a specific target protein. The electrophoresis membrane is washed in a solution containing the primary antibody, before excess antibody is washed off. A secondary antibody is added which recognizes and binds to the primary antibod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bird
Birds are a group of warm-blooded vertebrates constituting the class Aves (), characterised by feathers, toothless beaked jaws, the laying of hard-shelled eggs, a high metabolic rate, a four-chambered heart, and a strong yet lightweight skeleton. Birds live worldwide and range in size from the bee hummingbird to the ostrich. There are about ten thousand living species, more than half of which are passerine, or "perching" birds. Birds have whose development varies according to species; the only known groups without wings are the extinct moa and elephant birds. Wings, which are modified forelimbs, gave birds the ability to fly, although further evolution has led to the loss of flight in some birds, including ratites, penguins, and diverse endemic island species. The digestive and respiratory systems of birds are also uniquely adapted for flight. Some bird species of aquatic environments, particularly seabirds and some waterbirds, have further evolved for swim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosylated
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not always in chemistry), glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation (also 'non-enzymatic glycation' and 'non-enzymatic glycosylation') may refer to a non-enzymatic reaction (though in practice, 'glycation' often refers more specifically to Maillard-type reactions). Glycosylation is a form of co-translational and post-translational modification. Glycans serve a variety of structural and functional roles in membrane and secreted proteins. The majority of proteins synthesized in the rough endoplasmic reticulum undergo glycosylation. Glycosylation is also present in the cytoplasm and nucleus as the ''O''-GlcNAc modification. Aglycosylation is a feature of engineered antibodies to bypass glycosylation. Five c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)