|
Aminopeptidase
Aminopeptidases are enzymes that catalyze the cleavage of amino acids from the amino terminus ( N-terminus) of proteins or peptides (exopeptidases). They are widely distributed throughout the animal and plant kingdoms and are found in many subcellular organelles, in cytosol, and as membrane components. Aminopeptidases are used in essential cellular functions. Many, but not all, of these peptidases are zinc metalloenzymes. Some aminopeptidases are monomeric, and others are assemblies of relatively high mass (50 kDa) subunits. cDNA sequences are available for several aminopeptidases and a crystal structure of the open state of human endoplasmic reticulum Aminopeptidase 1 ERAP1 is presented here. Amino acid sequences determined directly or deduced from cDNAs indicate some amino acid sequence homologies in organisms as diverse as ''Escherichia coli'' and mammals, particularly in catalytically important residues or in residues involved in metal ion binding. One important aminope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alanine Aminopeptidase
Membrane alanyl aminopeptidase () also known as alanyl aminopeptidase (AAP) or aminopeptidase N (AP-N) is an enzyme that in humans is encoded by the ANPEP gene. Function Aminopeptidase N is located in the small-intestinal and renal microvillar membrane, and also in other plasma membranes. In the small intestine aminopeptidase N plays a role in the final digestion of peptides generated from hydrolysis of proteins by gastric and pancreatic proteases. Its function in proximal tubular epithelial cells and other cell types is less clear. The large extracellular carboxyterminal domain contains a pentapeptide consensus sequence characteristic of members of the zinc-binding metalloproteinase superfamily. Sequence comparisons with known enzymes of this class showed that CD13 and aminopeptidase N are identical. The latter enzyme was thought to be involved in the metabolism of regulatory peptides by diverse cell types, including small intestinal and renal tubular epithelial cells, macro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ARTS-1
Type 1 tumor necrosis factor receptor shedding aminopeptidase regulator, also known as endoplasmic reticulum aminopeptidase 1 (ARTS-1), is a protein which in humans is encoded by the ''ARTS-1'' gene. Endoplasmic reticulum amino peptidase 1 is active in the endoplasmic reticulum, which is involved in protein processing and transport. This protein is an aminopeptidase, which is an enzyme that cleaves peptides at the N-terminal into smaller fragments called amino acids. Nomenclature ARTS1 is also known as: * ER aminopeptidase 1 (ERAP1) the name accepted by the Hugo Gene Nomenclature Committee * ER aminopeptidase associated with antigen processing (ERAAP) in mice * Adipocyte-derived leucine aminopeptidase (ALAP) * Puromycin-insensitive leucine aminopeptidase (PILS-AP) Function ERAP1 has two major functions in the immune system: *First, ERAP1 cleaves several proteins called cytokine receptors on the surface of cells. Cleaving these receptors reduces their ability to transmit c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxypeptidase
A carboxypeptidase ( EC number 3.4.16 - 3.4.18) is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed. Functions Initial studies on carboxypeptidases focused on pancreatic carboxypeptidases A1, A2, and B in the digestion of food. Most carboxypeptidases are not, however, involved in catabolism. Instead they help to mature proteins, for example Post-translational modification. They also regulate biological processes, such as the biosynthesis of neuroendocrine peptides such as insulin requires a carboxypeptidase. Carboxypeptidases also function in blood clotting, growth factor production, wound healing, rep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins. Abundance It is estimated that approximately half of all proteins contain a metal. In another estimate, about one quarter to one third of all proteins are proposed to require metals to carry out their functions. Thus, metalloproteins have many different functions in cells, such as storage and transport of proteins, enzymes and signal transduction proteins, or infectious diseases. The abundance of metal binding proteins may be inherent to the amino acids that proteins use, as even artificial proteins without evolutionary history will readily bind metals. Most metals in the human body are bound to proteins. For instance, the relatively high concentration of iron in the human body is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enterokinase
Enteropeptidase (also called enterokinase) is an enzyme produced by cells of the duodenum and is involved in digestion in humans and other animals. Enteropeptidase converts trypsinogen (a zymogen) into its active form trypsin, resulting in the subsequent activation of pancreatic digestive enzymes. Absence of enteropeptidase results in intestinal digestion impairment. Enteropeptidase is a serine protease () consisting of a disulfide-linked heavy-chain of 82-140 kDa that anchors enterokinase in the intestinal brush border membrane and a light-chain of 35–62 kDa that contains the catalytic subunit. Enteropeptidase is a part of the chymotrypsin-clan of serine proteases, and is structurally similar to these proteins. Historical significance Enteropeptidase was discovered by Ivan Pavlov, who was awarded the 1904 Nobel Prize in Physiology or Medicine for his studies of gastrointestinal physiology. It is the first known enzyme to activate other enzymes, and it remains a remarkable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactase
Lactase is an enzyme produced by many organisms. It is located in the brush border of the small intestine of humans and other mammals. Lactase is essential to the complete digestion of whole milk; it breaks down lactose, a sugar which gives milk its sweetness. People who have deficiency of lactase, and consume dairy products, may experience the symptoms of lactose intolerance. Lactase can be purchased as a food supplement, and is added to milk to produce "lactose-free" milk products. Lactase (also known as lactase-phlorizin hydrolase, or LPH), a part of the β-galactosidase family of enzymes, is a glycoside hydrolase involved in the hydrolysis of the disaccharide lactose into constituent galactose and glucose monomers. Lactase is present predominantly along the brush border membrane of the differentiated enterocytes lining the villi of the small intestine. In humans, lactase is encoded by the LCT gene on chromosome 2. Uses Food use Lactase is an enzyme that some peo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sucrase
Sucrase is a digestive enzyme that catalyzes the hydrolysis of sucrose to its subunits fructose and glucose. One form, sucrase-isomaltase, is secreted in the small intestine on the brush border. The sucrase enzyme invertase, which occurs more commonly in plants, also hydrolyzes sucrose but by a different mechanism. Types * is isomaltase * is invertase * is sucrose alpha-glucosidase Physiology Sucrose intolerance (also known as congenital sucrase-isomaltase deficiency (CSID), genetic sucrase-isomaltase deficiency (GSID), or sucrase-isomaltase deficiency) occurs when sucrase is not being secreted in the small intestine. With sucrose intolerance, the result of consuming sucrose is excess gas production and often diarrhea and malabsorption. Lactose intolerance is a related disorder that reflects an individual's inability to hydrolyze the disaccharide lactose. Sucrase is secreted by the tips of the villi of the epithelium in the small intestine. Its levels are reduced in response ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltase
Maltase (, ''alpha-glucosidase'', ''glucoinvertase'', ''glucosidosucrase'', ''maltase-glucoamylase'', ''alpha-glucopyranosidase'', ''glucosidoinvertase'', ''alpha-D-glucosidase'', ''alpha-glucoside hydrolase'', ''alpha-1,4-glucosidase'', ''alpha-D-glucoside glucohydrolase'') is one type of alpha-glucosidase enzymes located in the brush border of the small intestine. This enzyme catalyzes the hydrolysis of disaccharide maltose into two simple sugars of glucose. Maltase is found in plants, bacteria, yeast, humans, and other vertebrates. It is thought to be synthesized by cells of the mucous membrane lining the intestinal wall. Digestion of starch requires six intestinal enzymes. Two of these enzymes are luminal endo-glucosidases named alpha-amylases. The other four enzymes have been identified as different maltases, exo-glucosidases bound to the luminal surface of enterocytes. Two of these maltase activities were associated with sucrase-isomaltase (maltase Ib, maltase Ia). The o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dipeptidases
Dipeptidases are enzymes secreted by enterocytes into the small intestine. Dipeptidases hydrolyze bound pairs of amino acids, called dipeptides. Dipeptidases are secreted onto the brush border of the villi in the small intestine, where they cleave dipeptides into their two component amino acids prior to absorption. They are also found within the enterocytes themselves, performing cytosolic digestion of absorbed dipeptides. Dipeptidases are exopeptidases, classified under EC number 3.4.13. See also * Membrane dipeptidase Membrane dipeptidase (, ''renal dipeptidase'', ''dehydropeptidase I (DPH I)'', ''dipeptidase'', ''aminodipeptidase'', ''dipeptide hydrolase'', ''dipeptidyl hydrolase'', ''nonspecific dipeptidase'', ''glycosyl-phosphatidylinositol-anchored renal di ... References External links * Enzymes EC 3.4.13 {{enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
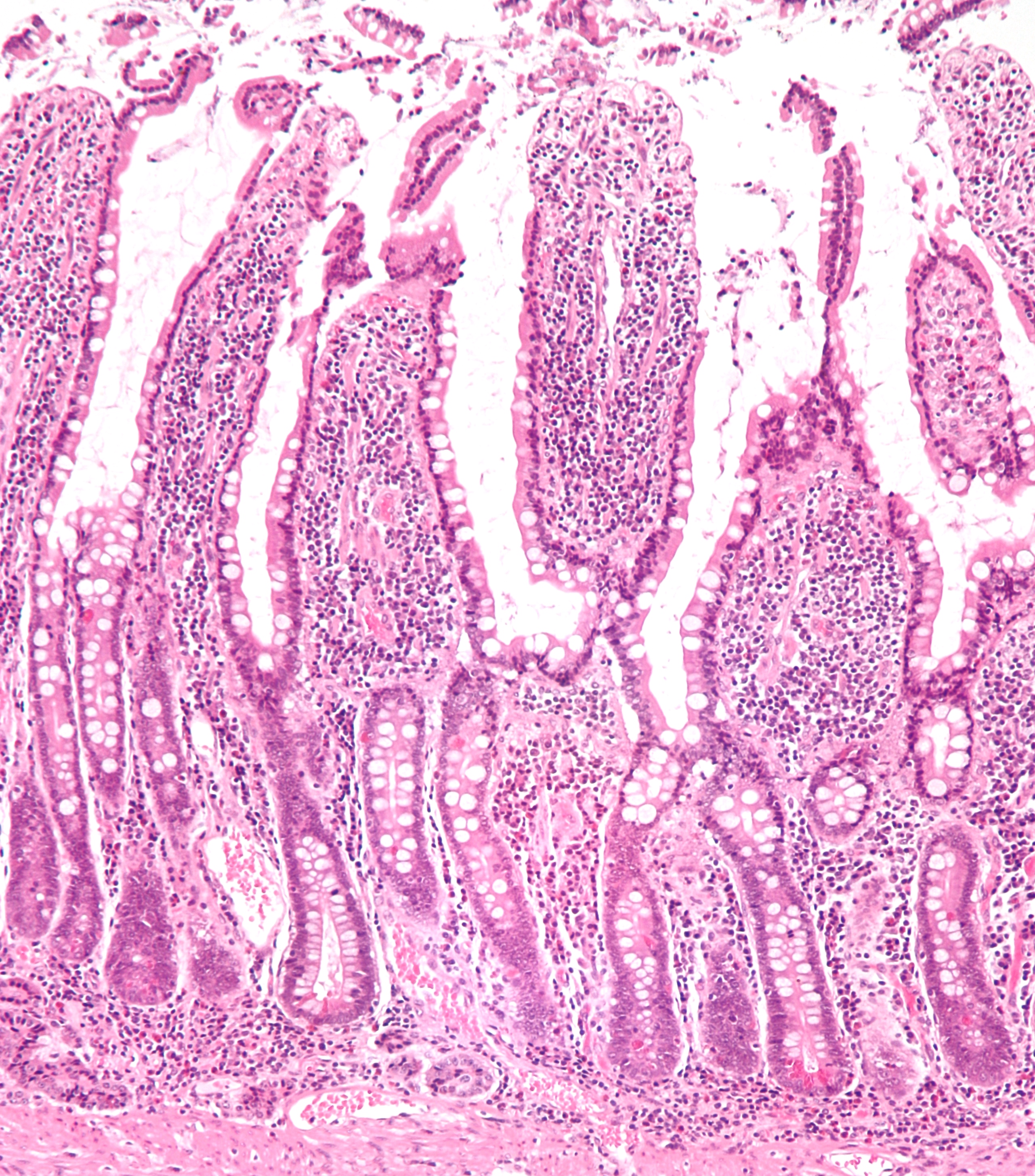
Small Intestine
The small intestine or small bowel is an organ (anatomy), organ in the human gastrointestinal tract, gastrointestinal tract where most of the #Absorption, absorption of nutrients from food takes place. It lies between the stomach and large intestine, and receives bile and pancreatic juice through the pancreatic duct to aid in digestion. The small intestine is about long and folds many times to fit in the abdomen. Although it is longer than the large intestine, it is called the small intestine because it is narrower in diameter. The small intestine has three distinct regions – the duodenum, jejunum, and ileum. The duodenum, the shortest, is where preparation for absorption through small finger-like protrusions called intestinal villus, villi begins. The jejunum is specialized for the absorption through its lining by enterocytes: small nutrient particles which have been previously digested by enzymes in the duodenum. The main function of the ileum is to absorb vitamin B12, vita ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |