|
2-butanol
2-Butanol, or ''sec''-butanol, is an organic compound with formula C H3CH( OH)CH2CH3. Its structural isomers are 1-butanol. isobutanol, and ''tert''-butanol. 2-Butanol is chiral and thus can be obtained as either of two stereoisomers designated as (''R'')-(−)-2-butanol and (''S'')-(+)-2-butanol. It is normally encountered as a 1:1 mixture of the two stereoisomers — a racemic mixture. This secondary alcohol is a flammable, colorless liquid that is soluble in three parts water and completely miscible with organic solvents. It is produced on a large scale, primarily as a precursor to the industrial solvent methyl ethyl ketone. Manufacture and applications 2-Butanol is manufactured industrially by the hydration of 1-butene or 2-butene: : Sulfuric acid is used as a catalyst for this conversion.. In the laboratory it can be prepared via Grignard reaction by reacting ethylmagnesium bromide with acetaldehyde in dried diethyl ether or tetrahydrofuran. Although some 2- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butanol
Butanol (also called butyl alcohol) is a four-carbon alcohol with a formula of C4 H9 O H, which occurs in five isomeric structures (four structural isomers), from a straight-chain primary alcohol to a branched-chain tertiary alcohol; all are a butyl or isobutyl group linked to a hydroxyl group (sometimes represented as BuOH, ''n''-BuOH, ''i''-BuOH, and ''t''-BuOH). These are ''n''-butanol, 2 stereoisomers of sec-butanol, isobutanol and ''tert''-butanol. Butanol is primarily used as a solvent and as an intermediate in chemical synthesis, and may be used as a fuel. Biologically produced butanol is called biobutanol, which may be ''n''-butanol or isobutanol. Isomers The unmodified term ''butanol'' usually refers to the straight chain isomer with the alcohol functional group at the terminal carbon, which is also known as ''n''-butanol or 1-butanol. The straight chain isomer with the alcohol at an internal carbon is ''sec''-butanol or 2-butanol. The branched isomer with the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutanol
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as ''i''-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either directly or as its esters. Its isomers are 1-butanol, 2-butanol, and ''tert''-butanol, all of which are important industrially. Production Isobutanol is produced by the carbonylation of propylene. Two methods are practiced industrially, hydroformylation is more common and generates a mixture of isobutyraldehyde and butyraldehyde: :CH3CH=CH2 + CO + H2 → CH3CH2CH2CHO The reaction is catalyzed by cobalt or rhodium complexes. The resulting aldehydes are hydrogenated to the alcohols, which are then separated. In Reppe carbonylation, the same products are obtained, but the hydrogenation is effected by the water-gas shift reaction.. Laboratory synthesis Propanol and methanol can be reacted to produce isobutyl alcohol via Guerbet con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutanol
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as ''i''-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either directly or as its esters. Its isomers are 1-butanol, 2-butanol, and ''tert''-butanol, all of which are important industrially. Production Isobutanol is produced by the carbonylation of propylene. Two methods are practiced industrially, hydroformylation is more common and generates a mixture of isobutyraldehyde and butyraldehyde: :CH3CH=CH2 + CO + H2 → CH3CH2CH2CHO The reaction is catalyzed by cobalt or rhodium complexes. The resulting aldehydes are hydrogenated to the alcohols, which are then separated. In Reppe carbonylation, the same products are obtained, but the hydrogenation is effected by the water-gas shift reaction.. Laboratory synthesis Propanol and methanol can be reacted to produce isobutyl alcohol via Guerbet con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-Butanol
''tert''-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as ''t''-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. ''tert''-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether. Natural occurrence ''tert''-Butyl alcohol has been identified in beer and chickpeas. It is also found in cassava, which is used as a fermentation ingredient in certain alcoholic beverages. Preparation ''tert''-Butyl alcohol is derived commercially from isobutane as a coproduct of propylene oxide production. It can also be produced by the catalytic hydration of isobutylene, or by a Grignard reaction between acetone and methylmagnesium chloride. Purification cannot be performed by simple distillation due to formation of an azeotrope with water, although initial drying of the solvent containing large amounts of water is performed by adding be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butanone
Butanone, also known as methyl ethyl ketone (MEK), is an organic compound with the formula CH3C(O)CH2CH3. This colourless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts.Wilhelm Neier, Guenter Strehlke "2-Butanone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran. Production Butanone may be produced by oxidation of 2-butanol. The dehydrogenation of 2-butanol is catalysed by copper, zinc, or bronze: :CH3CH(OH)CH2CH3 → CH3C(O)CH2CH3 + H2 This is used to produce approximately 700 million kilograms yearly. Other syntheses that have been examined but not implemented include Wacker oxidation of 2-butene and oxidation of isobutylbenzene, which is analogous to the industrial production of acetone. The cumene pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-butanol
''tert''-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as ''t''-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. ''tert''-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether. Natural occurrence ''tert''-Butyl alcohol has been identified in beer and chickpeas. It is also found in cassava, which is used as a fermentation ingredient in certain alcoholic beverages. Preparation ''tert''-Butyl alcohol is derived commercially from isobutane as a coproduct of propylene oxide production. It can also be produced by the catalytic hydration of isobutylene, or by a Grignard reaction between acetone and methylmagnesium chloride. Purification cannot be performed by simple distillation due to formation of an azeotrope with water, although initial drying of the solvent containing large amounts of water is performed by adding be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Ethyl Ketone
Butanone, also known as methyl ethyl ketone (MEK), is an organic compound with the formula CH3C(O)CH2CH3. This colourless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts.Wilhelm Neier, Guenter Strehlke "2-Butanone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran. Production Butanone may be produced by oxidation of 2-butanol. The dehydrogenation of 2-butanol is catalysed by copper, zinc, or bronze: :CH3CH(OH)CH2CH3 → CH3C(O)CH2CH3 + H2 This is used to produce approximately 700 million kilograms yearly. Other syntheses that have been examined but not implemented include Wacker oxidation of 2-butene and oxidation of isobutylbenzene, which is analogous to the industrial production of acetone. The cumene pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-butene
But-2-ene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting ''cis''/''trans''-isomerism (also known as (''E''/''Z'')-isomerism); that is, it exists as two geometric isomers ''cis''-but-2-ene ((''Z'')-but-2-ene) and ''trans-''but-2-ene ((''E'')-but-2-ene). It is a petrochemical, produced by the catalytic cracking of crude oil or the dimerization of ethylene. Its main uses are in the production of gasoline (petrol) and butadiene,. although some but-2-ene is also used to produce the solvent butanone via hydration to 2-butanol followed by oxidation. The two isomers are extremely difficult to separate by distillation because of the proximity of their boiling points (~4 °C for ''cis'' and ~1 °C for ''trans'' ). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydration Reaction
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed industrially to produce ethanol, isopropanol, and butan-2-ol.. Organic chemistry Epoxides to glycol Several million tons of ethylene glycol are produced annually by the hydration of oxirane, a cyclic compound also known as ethylene oxide: : C2H4O + H2O → HO–CH2CH2–OH Acid catalysts are typically used. Alkenes For the hydration of alkenes, the general chemical equation of the reaction is the following: :RRC=CH2 + H2O → RRC(OH)-CH3 A hydroxyl group (OH−) attaches to one carbon of the double bond, and a proton (H+) adds to the other. The reaction is highly exothermic. In the first step, the alkene acts as a nucleophile and attacks the proton, following Markovnikov's rule. In the second step an H2O molecule bonds to the other, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
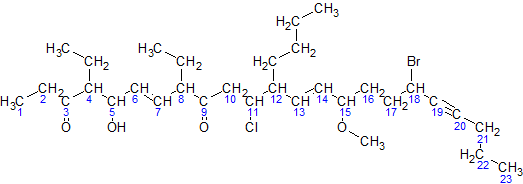
But-2-ene-hydration-2D-skeletal
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the ''Nomenclature of Organic Chemistry'' (informally called the Blue Book). Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry. To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when it is necessary to give an unambiguous and absolute definition to a compound. IUPAC names can sometimes be simpler than older names, as with ethanol, instead of ethyl alcohol. For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over. However, the common or trivial name is often substantially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |