|
-ene
The suffix -ene is used in organic chemistry to form names of organic compounds where the -C=C- group has been attributed the highest priority according to the rules of organic nomenclature. Sometimes a number between hyphens is inserted before it to say that the double bond is between that atom and the atom with the next number up. This suffix comes from the end of the word ethylene, which is the simplest alkene. The final "-e" disappears if it comes before by a suffix that starts with a vowel, e.g. "-enal", which is a compound that contains both a -C=C- bond and an aldehyde functional group. If the other suffix starts with a consonant or "y", the final "-e" remains, ''e.g.'' "-enediyne" (which has the "-ene" suffix and also the "-yne" suffix, for a compound with a double bond and two triple bonds.) A Greek number prefix before the "-ene" indicates how many double bonds there are in the compound, e.g. butadiene. The suffix "-ene" is also used in inorganic chemistry to indicate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
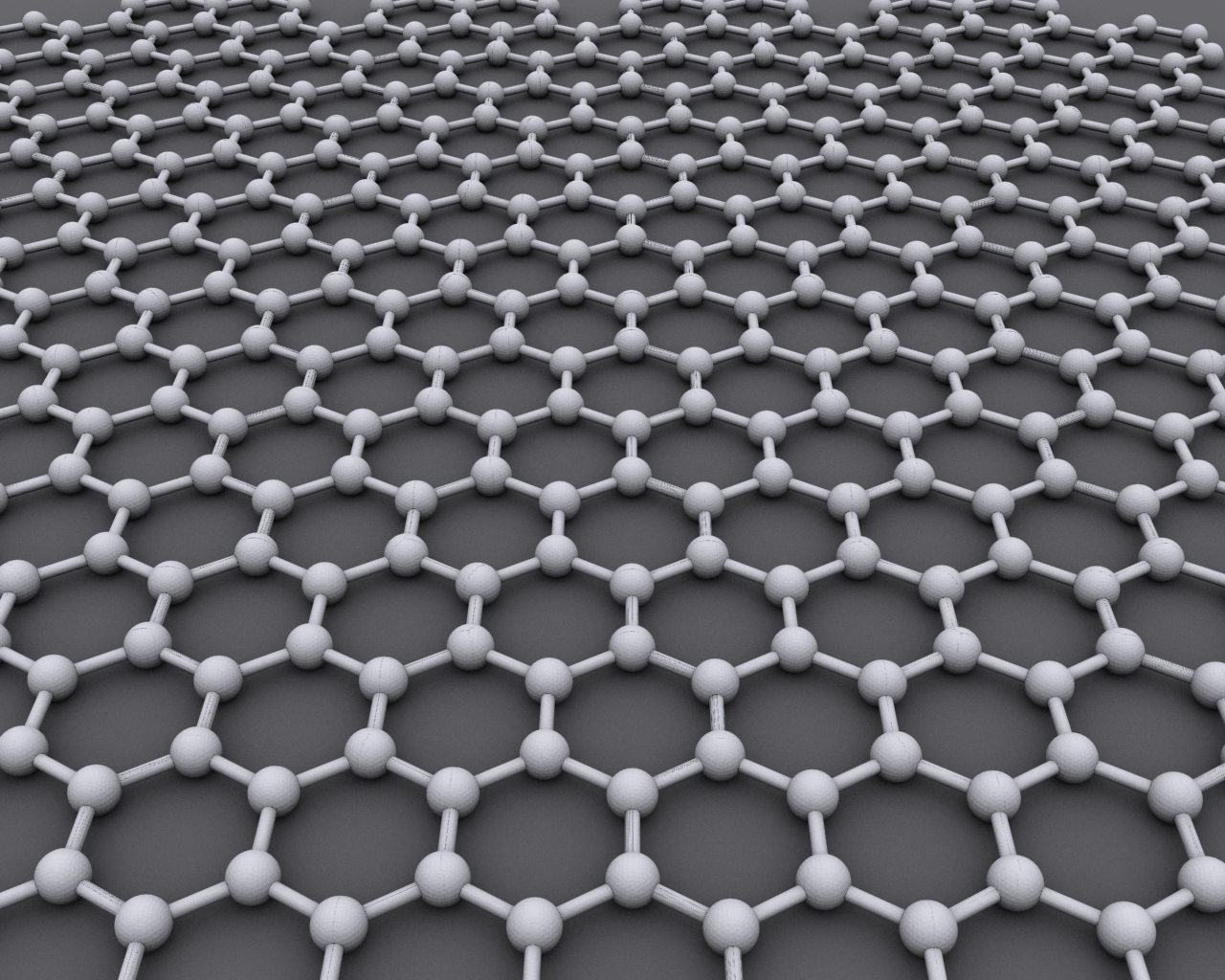
Graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure. "Carbon nanostructures for electromagnetic shielding applications", Mohammed Arif Poothanari, Sabu Thomas, et al., ''Industrial Applications of Nanomaterials'', 2019. "Carbon nanostructures include various low-dimensional allotropes of carbon including carbon black (CB), carbon fiber, carbon nanotubes (CNTs), fullerene, and graphene." The name is derived from "graphite" and the suffix -ene, reflecting the fact that the allotrope of carbon contains numerous double bonds. Each atom in a graphene sheet is connect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC Nomenclature Of Organic Chemistry
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the '' Nomenclature of Organic Chemistry'' (informally called the Blue Book). Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry. To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when it is necessary to give an unambiguous and absolute definition to a compound. IUPAC names can sometimes be simpler than older names, as with ethanol, instead of ethyl alcohol. For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over. However, the common or trivial name is often substantiall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Nomenclature
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the '' Nomenclature of Organic Chemistry'' (informally called the Blue Book). Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry. To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when it is necessary to give an unambiguous and absolute definition to a compound. IUPAC names can sometimes be simpler than older names, as with ethanol, instead of ethyl alcohol. For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over. However, the common or trivial name is often substantial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds). Ethylene is widely used in the chemical industry, and its worldwide production (over 150 million tonnes in 2016) exceeds that of any other organic compound. Much of this production goes toward polyethylene, a widely used plastic containing polymer chains of ethylene units in various chain lengths. Ethylene is also an important natural plant hormone and is used in agriculture to force the ripening of fruits. The hydrate of ethylene is ethanol. Structure and properties This hydrocarbon has four hydrogen atoms bound to a pair of carbon atoms that are connected by a double bond. All six atoms that comprise ethylene are coplanar. The H-C-H angle is 117.4°, close to the 120° for ideal sp² hybridized carbon. The molecule is also relatively weak: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Germanene
Germanene is a material made up of a single layer of germanium atoms. The material is created in a process similar to that of silicene and graphene, in which high vacuum and high temperature are used to deposit a layer of germanium atoms on a substrate. High-quality thin films of germanene have revealed unusual two-dimensional structures with novel electronic properties suitable for semiconductor device applications and materials science research. Preparation and structure In September 2014, G. Le Lay and others reported the deposition of a single atom thickness, ordered and two-dimensional multi-phase film by molecular beam epitaxy upon a gold surface in a crystal lattice with Miller indices (111). The structure was confirmed with scanning tunneling microscopy (STM) revealing a nearly flat honeycomb structure. Additional confirmation was obtained by spectroscopic measurement and density functional theory calculations. The development of high quality and nearly flat single atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stanene
Stanene is a topological insulator, theoretically predicted by Prof. Shoucheng Zhang's group at Stanford, which may display dissipationless currents at its edges near room temperature. It is composed of tin atoms arranged in a single layer, in a manner similar to graphene. Stanene got its name by combining ''stannum'' (the List of chemical element name etymologies, Latin name for tin) with the suffix ''-ene'' used by graphene. Research is ongoing in Germany and China, as well as at laboratories at Stanford and UCLA. The addition of fluorine atoms to the tin lattice could extend the critical temperature up to 100 °C. This would make it practical for use in integrated circuits to make smaller, faster and more energy efficient computers. See also *Graphene *Silicene * Boron Stannenes (Similar name to Stanene) *Stannane (similar name as Stanene, too) *Semiconductors *Topological Insulator *Superconductivity Superconductivity is a set of physical properties observed in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, the French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a materia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borophene
Borophene is a crystalline atomic monolayer of boron, i.e., it is a two-dimensional allotrope of boron and also known as ''boron sheet''. First predicted by theory in the mid-1990s, different borophene structures were experimentally confirmed in 2015. Properties Experimentally various atomically thin, crystalline and metallic borophenes were synthesized on clean metal surfaces under ultrahigh-vacuum conditions. Its atomic structure consists of mixed triangular and hexagonal motifs, such as shown in Figure 1. The atomic structure is a consequence of an interplay between two-center and multi-center in-plane bonding, which is typical for electron deficient elements like boron. Borophenes exhibit in-plane elasticity and ideal strength. It can be stronger than graphene, and more flexible, in some configurations. Boron nanotubes are also stiffer than graphene, with a higher 2D Young's modulus than any other known carbon and noncarbon nanostructures. Borophenes undergo novel str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicene
Silicene is a two-dimensional allotrope of silicon, with a hexagonal honeycomb structure similar to that of graphene. Contrary to graphene, silicene is not flat, but has a periodically buckled topology; the coupling between layers in silicene is much stronger than in multilayered graphene; and the oxidized form of silicene, 2D silica, has a very different chemical structure from graphene oxide. History Although theorists had speculated about the existence and possible properties of free-standing silicene, researchers first observed silicon structures that were suggestive of silicene in 2010. Using a scanning tunneling microscope they studied self-assembled silicene nanoribbons and silicene sheets deposited onto a silver crystal, Ag(110) and Ag(111), with atomic resolution. The images revealed hexagons in a honeycomb structure similar to that of graphene, which, however, were shown to originate from the silver surface mimicking the hexagons. Density functional theory (DFT) cal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture. Key concepts Many inorganic compounds are ionic compounds, consisting of cations and anions joined by ionic bonding. Examples of salts (which are ionic compounds) are magnesium chloride MgCl2, which consists of magnesium cations Mg2+ and chloride anions Cl−; or sodium oxide Na2O, which consists of sodium cations Na+ and oxide anions O2−. In any salt, the proportions of the ions are such that the electric charges cancel out, so that the bulk compound is ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affix
In linguistics, an affix is a morpheme that is attached to a word stem to form a new word or word form. Affixes may be derivational, like English ''-ness'' and ''pre-'', or inflectional, like English plural ''-s'' and past tense ''-ed''. They are bound morphemes by definition; prefixes and suffixes may be separable affixes. Affixation is the linguistic process that speakers use to form different words by adding morphemes at the beginning (prefixation), the middle (infixation) or the end (suffixation) of words. Positional categories of affixes ''Prefix'' and ''suffix'' may be subsumed under the term ''adfix'', in contrast to ''infix.'' When marking text for interlinear glossing, as in the third column in the chart above, simple affixes such as prefixes and suffixes are separated from the stem with hyphens. Affixes which disrupt the stem, or which themselves are discontinuous, are often marked off with angle brackets. Reduplication is often shown with a tilde. Affixes whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |