Δ34S on:
[Wikipedia]
[Google]
[Amazon]
 The δ34S (pronounced ''delta 34 S'') value is a standardized method for reporting measurements of the ratio of two stable
The δ34S (pronounced ''delta 34 S'') value is a standardized method for reporting measurements of the ratio of two stable
 Sulfur from meteorites was determined in the early 1950s to be an adequate reference standard because it exhibited a small variability in isotopic ratios. It was also believed that because of their extraterrestrial provenances, meteors represented primordial terrestrial isotopic conditions. During a meeting of the
Sulfur from meteorites was determined in the early 1950s to be an adequate reference standard because it exhibited a small variability in isotopic ratios. It was also believed that because of their extraterrestrial provenances, meteors represented primordial terrestrial isotopic conditions. During a meeting of the
 Two mechanisms of
Two mechanisms of
 The δ34S (pronounced ''delta 34 S'') value is a standardized method for reporting measurements of the ratio of two stable
The δ34S (pronounced ''delta 34 S'') value is a standardized method for reporting measurements of the ratio of two stable isotopes of sulfur
Sulfur (16S) has 23 known isotopes with mass numbers ranging from 27 to 49, four of which are stable: 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%). The preponderance of sulfur-32 is explained by its production from carbon-12 plus suc ...
, 34S:32S, in a sample against the equivalent ratio in a known reference standard. Presently, the most commonly used standard is Vienna-Canyon Diablo Troilite (VCDT). Results are reported as variations from the standard ratio in parts per thousand, per mil
Per mille (from Latin , "in each thousand") is an expression that means parts per thousand. Other recognised spellings include per mil, per mill, permil, permill, or permille.
The associated sign is written , which looks like a percent si ...
or ''per mille'', using the ‰ symbol. Heavy and light sulfur isotopes fractionate at different rates and the resulting δ34S values, recorded in marine sulfate or sedimentary sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds l ...
s, have been studied and interpreted as records of the changing sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a consti ...
throughout the earth's history.
Calculation
Of the 25 knownisotopes of sulfur
Sulfur (16S) has 23 known isotopes with mass numbers ranging from 27 to 49, four of which are stable: 32S (95.02%), 33S (0.75%), 34S (4.21%), and 36S (0.02%). The preponderance of sulfur-32 is explained by its production from carbon-12 plus suc ...
, four are stable
A stable is a building in which livestock, especially horses, are kept. It most commonly means a building that is divided into separate stalls for individual animals and livestock. There are many different types of stables in use today; the ...
. In order of their abundance, those isotopes are 32S (94.93%), 34S (4.29%), 33S (0.76%), and 36S (0.02%). The δ34S value refers to a measure of the ratio of the two most common stable sulfur isotopes, 34S:32S, as measured in a sample against that same ratio as measured in a known reference standard. The lowercase delta character is used by convention, to be consistent with use in other areas of stable isotope chemistry. That value can be calculated in per mil
Per mille (from Latin , "in each thousand") is an expression that means parts per thousand. Other recognised spellings include per mil, per mill, permil, permill, or permille.
The associated sign is written , which looks like a percent si ...
(‰, parts per thousand) as:
: ‰
Less commonly, if the appropriate isotope abundances are measured, similar formulae can be used to quantify ratio variations between 33S and 32S, and 36S and 32S, reported as δ33S and δ36S, respectively.
Reference standard
National Science Foundation
The National Science Foundation (NSF) is an independent agency of the United States government that supports fundamental research and education in all the non-medical fields of science and engineering. Its medical counterpart is the National ...
in April 1962, troilite from the Canyon Diablo meteorite
The Canyon Diablo meteorite refers to the many fragments of the asteroid that created Meteor Crater (also called Barringer Crater), Arizona, United States. Meteorites have been found around the crater rim, and are named for nearby Canyon Diab ...
found in Arizona, US, was established as the standard with which δ34S values (and other sulfur stable isotopic ratios) could be calculated. Known as Canyon Diablo Troilite (CDT), the standard was established as having a 32S:34S ratio of 22.220 and was used for around three decades. In 1993, the International Atomic Energy Agency
The International Atomic Energy Agency (IAEA) is an intergovernmental organization that seeks to promote the peaceful use of nuclear energy and to inhibit its use for any military purpose, including nuclear weapons. It was established in 19 ...
(IAEA) established a new standard, Vienna-CDT (VCDT), based on artificially prepared silver sulfide
Silver sulfide is an inorganic compound with the formula . A dense black solid, it is the only sulfide of silver. It is useful as a photosensitizer in photography. It constitutes the tarnish that forms over time on silverware and other silver ob ...
(IAEA-S-1) that was defined to have a δ34SVCDT value of −0.3‰. In 1994, the original CDT material was found not to be isotopically homogeneous, with internal variations as great as 0.4‰, confirming its unsuitability as a reference standard.
Causes of variations
 Two mechanisms of
Two mechanisms of fractionation
Fractionation is a separation process in which a certain quantity of a mixture (of gases, solids, liquids, enzymes, or isotopes, or a suspension) is divided during a phase transition, into a number of smaller quantities (fractions) in which the ...
occur that alter sulfur stable isotope ratios: kinetic effects, especially due to the metabolism of sulfate-reducing bacteria
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate () as termin ...
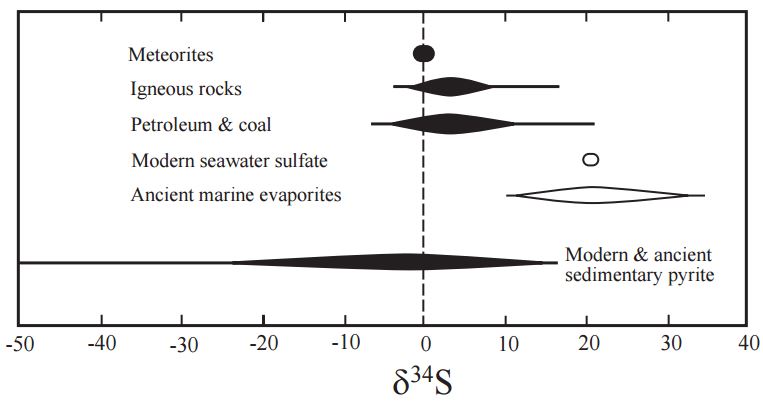
, and isotope exchange reactions that occur between sulfide phases based on temperature. With VCDT as the reference standard, natural δ34S value variations have been recorded between -72‰ and +147‰.
The presence of sulfate-reducing bacteria, which reduce
Reduction, reduced, or reduce may refer to:
Science and technology Chemistry
* Reduction (chemistry), part of a reduction-oxidation (redox) reaction in which atoms have their oxidation state changed.
** Organic redox reaction, a redox reacti ...
sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and man ...
() to hydrogen sulfide
Hydrogen sulfide is a chemical compound with the formula . It is a colorless chalcogen-hydride gas, and is poisonous, corrosive, and flammable, with trace amounts in ambient atmosphere having a characteristic foul odor of rotten eggs. The unde ...
(H2S), has played a significant role in the oceanic δ34S value throughout the earth's history. Sulfate-reducing bacteria metabolize 32S more readily than 34S, resulting in an increase in the value of the δ34S in the remaining sulfate in the seawater. Archean
The Archean Eon ( , also spelled Archaean or Archæan) is the second of four geologic eons of Earth's history, representing the time from . The Archean was preceded by the Hadean Eon and followed by the Proterozoic.
The Earth during the Arch ...
pyrite
The mineral pyrite (), or iron pyrite, also known as fool's gold, is an iron sulfide with the chemical formula Fe S2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral.
Pyrite's metallic luster and pale brass-yellow hue ...
found in barite
Baryte, barite or barytes ( or ) is a mineral consisting of barium sulfate ( Ba S O4). Baryte is generally white or colorless, and is the main source of the element barium. The ''baryte group'' consists of baryte, celestine (strontium sulfate), ...
in the Warrawoona Group, Western Australia, with sulfur fractionations as great as 21.1‰ hint at the presence of sulfate-reducers as early as .
The δ34S value, recorded by sulfate in marine evaporite
An evaporite () is a water-soluble sedimentary mineral deposit that results from concentration and crystallization by evaporation from an aqueous solution. There are two types of evaporite deposits: marine, which can also be described as ocea ...
s, can be used to chart the sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a consti ...
throughout earth's history. The Great Oxygenation Event
The Great Oxidation Event (GOE), also called the Great Oxygenation Event, the Oxygen Catastrophe, the Oxygen Revolution, the Oxygen Crisis, or the Oxygen Holocaust, was a time interval during the Paleoproterozoic era when the Earth's atmosphere ...
around altered the sulfur cycle radically, as increased atmospheric oxygen permitted an increase in the mechanisms that could fractionate sulfur isotopes, leading to an increase in the δ34S value from ~0‰ pre-oxygenation. Approximately , the δ34S values in seawater sulfates began to vary more and those in sedimentary sulfates grew more negative. Researchers have interpreted this excursion as indicative of an increase in water column
A water column is a conceptual column of water from the surface of a sea, river or lake to the bottom sediment.Munson, B.H., Axler, R., Hagley C., Host G., Merrick G., Richards C. (2004).Glossary. ''Water on the Web''. University of Minnesota- ...
oxygenation with continued periods of anoxia in the deepest waters. Modern seawater sulfate δ34S values are consistently 21.0 ± 0.2‰ across the world's oceans, while sedimentary sulfides vary widely. Seawater sulfate δ34S and δ18O values exhibit similar trends not seen in sedimentary sulfide minerals.
See also
* * *Isotopic signature
An isotopic signature (also isotopic fingerprint) is a ratio of non-radiogenic ' stable isotopes', stable radiogenic isotopes, or unstable radioactive isotopes of particular elements in an investigated material. The ratios of isotopes in a sample ...
* Isotope analysis
Isotope analysis is the identification of isotopic signature, abundance of certain stable isotopes of chemical elements within organic and inorganic compounds. Isotopic analysis can be used to understand the flow of energy through a food we ...
* Isotope geochemistry
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various elements. Variations in isotopic abundance are measured by isotope ratio mass spectrometry, and can reveal i ...
References
Citations * {{DEFAULTSORT:Delta34S Isotopes of sulfur Environmental isotopes Geochemistry Bioindicators