transition metal carbene complex on:
[Wikipedia]
[Google]
[Amazon]
A transition metal carbene complex is an organometallic compound featuring a divalent organic
 ''N''-Heterocyclic carbenes (NHCs) are particularly common carbene ligands. They are popular because they are more readily prepared than Schrock and Fischer carbenes. In fact many NHCs are isolated as the free ligand, since they are persistent carbenes. Being strongly stabilized by π-donating substituents, NHCs are powerful σ-donors but π-bonding with the metal is weak. For this reason, the bond between the carbon and the metal center is often represented by a single dative bond, whereas Fischer and Schrock carbenes are usually depicted with double bonds to metal. Continuing with this analogy, NHCs are often compared with trialkyl
''N''-Heterocyclic carbenes (NHCs) are particularly common carbene ligands. They are popular because they are more readily prepared than Schrock and Fischer carbenes. In fact many NHCs are isolated as the free ligand, since they are persistent carbenes. Being strongly stabilized by π-donating substituents, NHCs are powerful σ-donors but π-bonding with the metal is weak. For this reason, the bond between the carbon and the metal center is often represented by a single dative bond, whereas Fischer and Schrock carbenes are usually depicted with double bonds to metal. Continuing with this analogy, NHCs are often compared with trialkyl
 The characterization of in the 1960s is often cited as the starting point of the area, although carbenoid ligands had been previously implicated. Ernst Otto Fischer, for this and other achievements in organometalic chemistry, was awarded the 1973
The characterization of in the 1960s is often cited as the starting point of the area, although carbenoid ligands had been previously implicated. Ernst Otto Fischer, for this and other achievements in organometalic chemistry, was awarded the 1973
ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
. The divalent organic ligand coordinated to the metal center is called a carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" m ...
. Carbene complexes for almost all transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s have been reported. Many methods for synthesizing them and reactions utilizing them have been reported. The term carbene ligand is a formalism since many are not derived from carbenes and almost none exhibit the reactivity characteristic of carbenes. Described often as , they represent a class of organic ligands intermediate between alkyls and carbynes . They feature in some catalytic reactions, especially alkene metathesis, and are of value in the preparation of some fine chemicals.
Classification
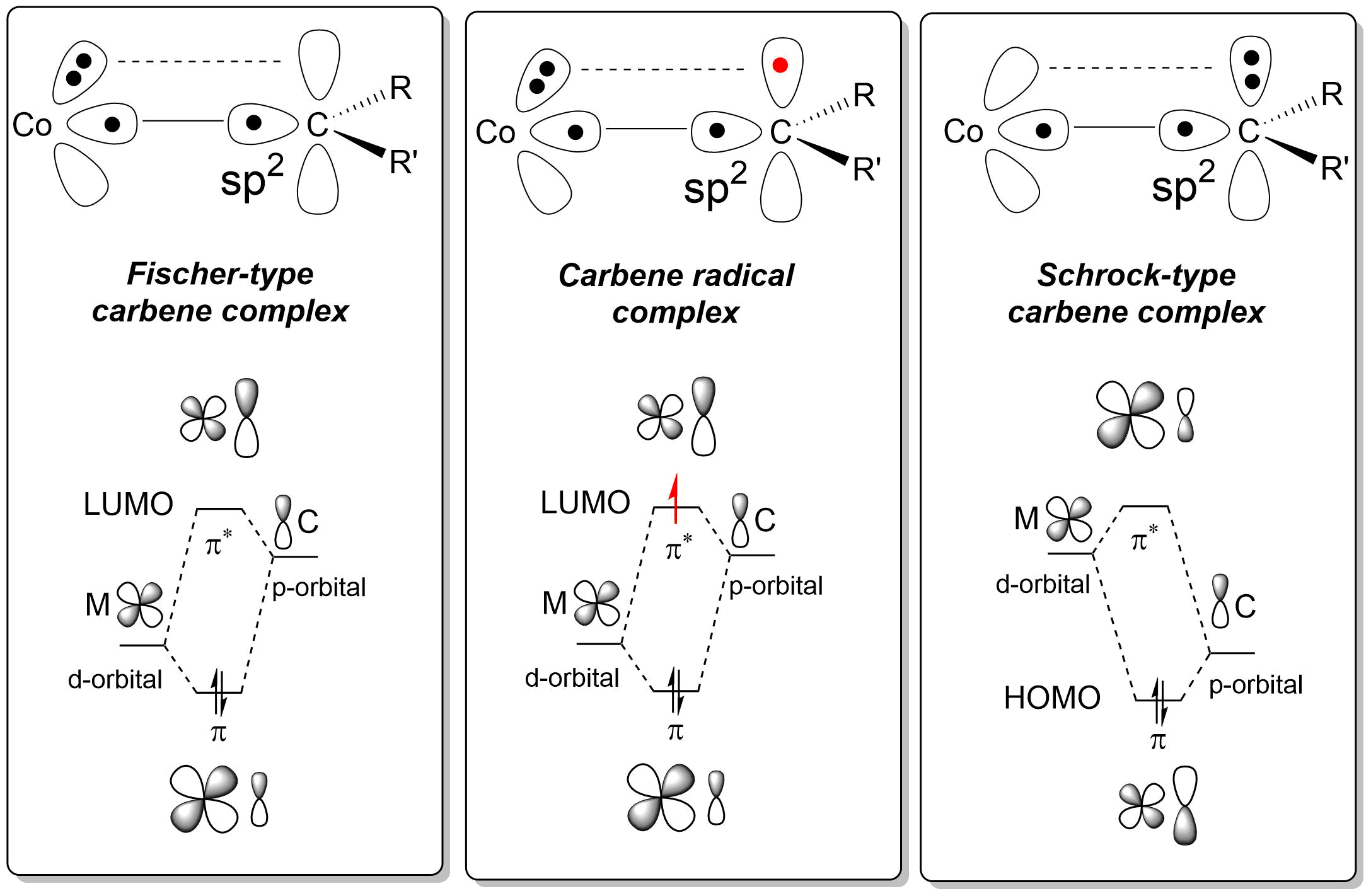
Metal carbene complexes are often classified into two types. The Fischer carbenes named after Ernst Otto Fischer feature strong π-acceptors at the metal and beingelectrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
at the carbene carbon atom. Schrock carbenes, named after Richard R. Schrock, are characterized by more nucleophilic carbene carbon centers; these species typically feature higher valent metals. ''N''-Heterocyclic carbenes (NHCs) were popularized following Arduengo's isolation of a stable free carbene in 1991. Reflecting the growth of the area, carbene complexes are now known with a broad range of different reactivities and diverse substituents. Often it is not possible to classify a carbene complex with regards to its electrophilicity or nucleophilicity.
Fischer carbenes
Fischer carbenes are found with: * lowoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
metal center
* middle and late transition metals Fe(0), Mo(0), Cr(0)
* π-acceptor metal ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
s
* π-donor substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as '' side ...
s on the carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" m ...
atom such as alkoxy and alkylated amino groups.
The chemical bonding (''Scheme 1'') is based on σ-type electron donation of the filled lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC '' Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. L ...
orbital of the carbene C atom to an empty metal d-orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any sp ...
, and π back bonding of a filled metal d-orbital to the empty p-orbital on the carbon atom. An example is the complex .
Fischer carbenes can be likened to ketones, with the carbene carbon atom being electrophilic, much like the carbonyl carbon atom of a ketone. Like ketones, Fischer carbene species can undergo aldol-like reactions. The hydrogen atoms attached to the carbon atom α to the carbene carbon atom are acidic, and can be deprotonated by a base such as ''n''-butyllithium, to give a nucleophile, which can undergo further reaction.
This carbene is the starting material for other reactions such as the Wulff-Dötz reaction.
Schrock carbenes
Schrock carbenes do not have π-accepting ligands. These complexes are nucleophilic at the carbene carbon atom. Schrock carbenes are typically found with: * highoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
metal center
* early transition metals Ti(IV), Ta(V)
* π-donor ligands
* hydrogen and alkyl substituents on carbenoid carbon.
Bonding in such complexes can be viewed as the coupling of a triplet state metal and triplet carbene. These bonds are polarized towards carbon and therefore the carbene atom is a nucleophile. An example of a Schrock carbene is the compound , with a tantalum(V) center doubly bonded to a neopentylidene ligand as well as three neopentyl ligands. An example of interest in organic synthesis is Tebbe's reagent.
''N''-Heterocyclic carbenes
: ''N''-Heterocyclic carbenes (NHCs) are particularly common carbene ligands. They are popular because they are more readily prepared than Schrock and Fischer carbenes. In fact many NHCs are isolated as the free ligand, since they are persistent carbenes. Being strongly stabilized by π-donating substituents, NHCs are powerful σ-donors but π-bonding with the metal is weak. For this reason, the bond between the carbon and the metal center is often represented by a single dative bond, whereas Fischer and Schrock carbenes are usually depicted with double bonds to metal. Continuing with this analogy, NHCs are often compared with trialkyl
''N''-Heterocyclic carbenes (NHCs) are particularly common carbene ligands. They are popular because they are more readily prepared than Schrock and Fischer carbenes. In fact many NHCs are isolated as the free ligand, since they are persistent carbenes. Being strongly stabilized by π-donating substituents, NHCs are powerful σ-donors but π-bonding with the metal is weak. For this reason, the bond between the carbon and the metal center is often represented by a single dative bond, whereas Fischer and Schrock carbenes are usually depicted with double bonds to metal. Continuing with this analogy, NHCs are often compared with trialkylphosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
ligands. Like phosphines, NHCs serve as spectator ligand In coordination chemistry, a spectator ligand is a ligand that does not participate in chemical reactions of the complex. Instead, spectator ligands (vs "actor ligands") occupy coordination sites. Spectator ligands tend to be of polydentate, such ...
s that influence catalysis through a combination of electronic and steric effects, but they do not directly bind substrates. Carbenes without a metal ligand have been produced in the lab.
Carbene radicals
Carbene radicals are long-lived reaction intermediates found with: * lowoxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
metal center with singly occupied dz2 orbital
* middle and late transition metal, e.g. Co(II)
* σ-donor and π-acceptor ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
* π-acceptor substituents on the ligand such as carbonyl or sulfonyl groups. The chemical bond present in carbene radicals is described as aspects of both Fischer and Schrock carbenes.

Applications of carbene complexes
The main applications of metal carbenes involves none of the above classes of compounds, but ratherheterogeneous catalyst
In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. ...
s used for alkene metathesis in the Shell higher olefin process. A variety of related reactions are used to interconvert light alkenes, e.g. butenes, propylene, and ethylene. Carbene-complexes are invoked as intermediates in the Fischer–Tropsch route to hydrocarbons. A variety of soluble carbene reagents, especially the Grubbs' and molybdenum-imido catalysts have been applied to laboratory-scale synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
* Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organ ...
of natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical sy ...
s and materials science. In the nucleophilic abstraction reaction, a methyl group can be abstracted from a Fischer carbene for further reaction.
Diazo compounds like methyl phenyldiazoacetate can be used for cyclopropanation or to insert into C-H bonds of organic substrates. These reactions are catalyzed by dirhodium tetraacetate or related chiral derivatives. Such catalysis is assumed to proceed via the intermediacy of carbene complexes.
History
:Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
.
See also
* Carbyne * Carbene radicalReferences
{{Coordination complexes Organometallic chemistry Carbenes Transition metals Coordination chemistry