Tetrahedrane on:
[Wikipedia]
[Google]
[Amazon]
Tetrahedrane is a hypothetical

 The tetrahedrane motif occurs broadly in chemistry.
The tetrahedrane motif occurs broadly in chemistry.
platonic hydrocarbon
In organic chemistry, a Platonic hydrocarbon is a hydrocarbon (molecule) whose structure matches one of the five Platonic solids, with carbon atoms replacing its vertices, carbon–carbon bonds replacing its edges, and hydrogen atoms as needed ...
with chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbol ...
and a tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the ...
structure. The molecule would be subject to considerable angle strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are ...
and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term ''tetrahedranes'' is used to describe a class of molecules and ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s with related structure, e.g. white phosphorus
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.
White phosphorus
White ...
.
Organic tetrahedranes
In 1978, Günther Maier prepared tetra- ''tert''-butyl-tetrahedrane. These bulky substituents envelop the tetrahedrane core. Maier suggested that bonds in the core are prevented from breaking because this would force the substituents closer together (corset effect) resulting in Van der Waals strain. Tetrahedrane is one of the possible platonic hydrocarbons and has theIUPAC name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among the possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choo ...
tricyclo .1.0.02,4utane.
Unsubstituted tetrahedrane () remains elusive, although it is predicted to be kinetically stable. One strategy that has been explored (but thus far failed) is reaction of propene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula CH3CH=CH2. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petro ...
with atomic carbon. Locking away a tetrahedrane molecule inside a fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
has only been attempted '' in silico''. Due to its bond strain and stoichiometry, tetranitrotetrahedrane has potential as a high-performance energetic material (explosive). Some properties have been calculated based on quantum chemical methods.
Tetra-''tert''-butyltetrahedrane
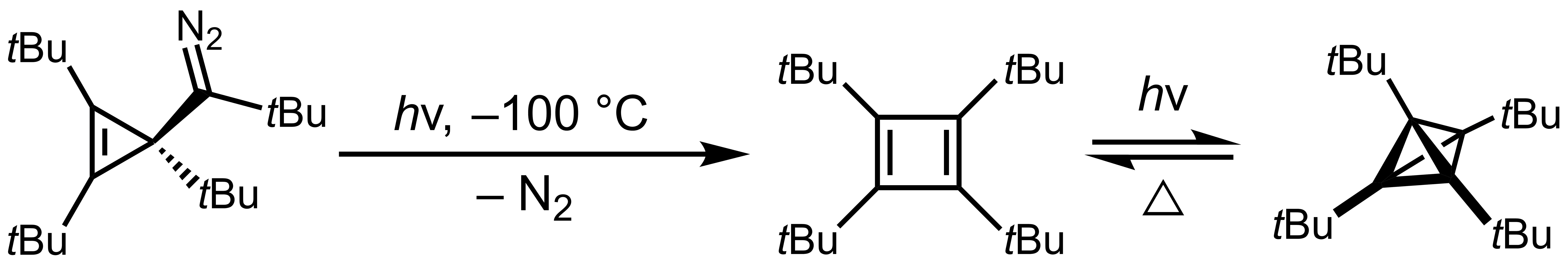
This compound was first synthesised starting from acycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". T ...
of an alkyne with t-Bu substituted maleic anhydride
Maleic anhydride is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and pol ...
, followed by rearrangement with carbon dioxide expulsion to a cyclopentadienone
Cyclopentadienone is an organic compound with molecular formula C5H4O. The parent cyclopentadienone is rarely encountered, because it rapidly dimerizes. Many substituted derivatives are known, notably tetraphenylcyclopentadienone. Such compounds ...
and its bromination
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polyme ...
, followed by addition of the fourth t-Bu group. Photochemical cheletropic elimination of carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
of the cyclopentadienone gives the target. Heating tetra-''tert''-butyltetrahedrane gives tetra-''tert''-butylcyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is ...
. Though the synthesis appears short and simple, by Maier's own account, it took several years of careful observation and optimization to develop the correct conditions for the challenging reactions to take place. For instance, the synthesis of tetrakis(''t-''butyl)cyclopentadienone from the tris(''t''-butyl)bromocyclopentadienone (itself synthesized with much difficulty) required over 50 attempts before working conditions could be found. The synthesis was described as requiring "astonishing persistence and experimental skill" in one retrospective of the work. In a classic reference work on stereochemistry, the authors remark that "the relatively straightforward scheme shown ..conceals both the limited availability of the starting material and the enormous amount of work required in establishing the proper conditions for each step."
:
Eventually, a more scalable synthesis was conceived, in which the last step was the photolysis of a cyclopropenyl-substituted diazomethane, which affords the desired product through the intermediacy of tetrakis(''tert''-butyl)cyclobutadiene: This approach took advantage of the observation that the tetrahedrane and the cyclobutadiene could be interconverted (uv irradiation in the forward direction, heat in the reverse direction).
:
Tetrakis(trimethylsilyl)tetrahedrane
Tetrakis(trimethylsilyl)tetrahedrane can be prepared by treatment of the cyclobutadiene precursor with tris(pentafluorophenyl)borane and is far more stable than the ''tert''-butyl analogue. The silicon–carbonbond
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Chemical bond, the attraction of atoms, ions or molecules to form chemica ...
is longer than a carbon–carbon bond, and therefore the corset effect is reduced. Whereas the ''tert''-butyl tetrahedrane melts at 135 °C concomitant with rearrangement to the cyclobutadiene, tetrakis(trimethylsilyl)tetrahedrane, which melts at 202 °C, is stable up to 300 °C, at which point it cracks to bis(trimethylsilyl)acetylene.
The tetrahedrane skeleton is made up of banana bonds, and hence the carbon atoms are high in s-orbital character. From NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
, sp- hybridization can be deduced, normally reserved for triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple bonds are stronger than the equivalent single bonds or double bonds, with a bond order o ...
s. As a consequence the bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
s are unusually short with 152 picometer
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer (American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth of ...
s.
Reaction with methyllithium
Methyllithium is the simplest organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in so ...
with tetrakis(trimethylsilyl)tetrahedrane yields tetrahedranyllithium. Coupling reactions A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = ...
with this lithium compound gives extended structures.
A bis(tetrahedrane) has also been reported. The connecting bond is even shorter with 143.6 pm. An ordinary carbon–carbon bond has a length of 154 pm.
:
Tetrahedranes with non-carbon cores
In tetrasilatetrahedrane features a core of foursilicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
atoms. The standard silicon–silicon bond is much longer (235 pm) and the cage is again enveloped by a total of 16 trimethylsilyl groups, which confer stability. The silatetrahedrane can be reduced with potassium graphite
Graphite intercalation compounds are complex materials having a formula where the ion or is inserted ( intercalated) between the oppositely charged carbon layers. Typically ''m'' is much less than 1. These materials are deeply colored solids t ...
to the tetrasilatetrahedranide potassium derivative. In this compound one of the silicon atoms of the cage has lost a silyl substituent and carries a negative charge. The potassium cation can be sequestered by a crown ether
In organic chemistry, crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups (). The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., . Impo ...
, and in the resulting complex potassium and the silyl anion are separated by a distance of 885 pm. One of the Si−–Si bonds is now 272 pm and its silicon atom has an inverted tetrahedral geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−) = 109.4712206...° ≈ 109.5° when all four substituents are ...
. Furthermore, the four cage silicon atoms are equivalent on the NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with ...
timescale due to migrations of the silyl substituents over the cage.
:
The dimerization reaction observed for the carbon tetrahedrane compound is also attempted for a tetrasilatetrahedrane. In this tetrahedrane the cage is protected by four so-called supersilyl groups in which a silicon atom has 3 ''tert''-butyl substituents. The dimer does not materialize but a reaction with iodine in benzene followed by reaction with the tri-''tert''-butylsilaanion results in the formation of an eight-membered silicon cluster compound
In chemistry, an atom cluster (or simply cluster) is an ensemble of bound atoms or molecules that is intermediate in size between a simple molecule and a nanoparticle; that is, up to a few nanometers (nm) in diameter. The term ''microcluster' ...
which can be described as a dumbbell (length 229 pm and with inversion of tetrahedral geometry) sandwiched between two almost-parallel rings.
:
In eight-membered clusters of in the same carbon group
The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.
In modern IUPAC notation, it is called group 14. In the field of sem ...
, tin
Tin is a chemical element with the symbol Sn (from la, stannum) and atomic number 50. Tin is a silvery-coloured metal.
Tin is soft enough to be cut with little force and a bar of tin can be bent by hand with little effort. When bent, t ...
and germanium the cluster atoms are located on the corners of a cube.
Inorganic and organometallic tetrahedranes
 The tetrahedrane motif occurs broadly in chemistry.
The tetrahedrane motif occurs broadly in chemistry. White phosphorus
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.
White phosphorus
White ...
(P4) and yellow arsenic (As4) are examples. Several metal carbonyl clusters are referred to as tetrahedranes, e.g. tetrarhodium dodecacarbonyl
Tetrarhodium dodecacarbonyl is the chemical compound with the formula Rh4(CO)12. This dark-red crystalline solid is the smallest binary rhodium carbonyl that can be handled as a solid under ambient conditions. It is used as a catalyst in organic ...
.
Metallatetrahedranes with a single metal (or phosphorus atom) capping a cyclopropyl trianion also exist.
*Organometallics 2019, 38, 21, 4054–4059.
*Organometallics 1984, 3, 1574−1583.
*Organometallics 1986, 5, 25−33.
*J. Am. Chem. Soc. 1984, 106, 3356−3357.
*J. Chem. Soc., Chem. Commun. 1984, 485−486.
*Science Advances 25 Mar 2020: Vol. 6, no. 13, doi:10.1126/sciadv.aaz3168
See also
*Dodecahedrane
Dodecahedrane is a chemical compound, a hydrocarbon with formula , whose carbon atoms are arranged as the vertices (corners) of a regular dodecahedron. Each carbon is bound to three neighbouring carbon atoms and to a hydrogen atom. This compound ...
*Prismane
Prismane or 'Ladenburg benzene' is a polycyclic hydrocarbon with the formula C6H6. It is an isomer of benzene, specifically a valence isomer. Prismane is far less stable than benzene. The carbon (and hydrogen) atoms of the prismane molecule are ...
* Prismane
References
{{reflist, 30em Cycloalkanes Cluster chemistry Hypothetical chemical compounds Tricyclic compounds