Sulfur Vulcanization on:
[Wikipedia]
[Google]
[Amazon]
 Sulfur vulcanization is a
Sulfur vulcanization is a
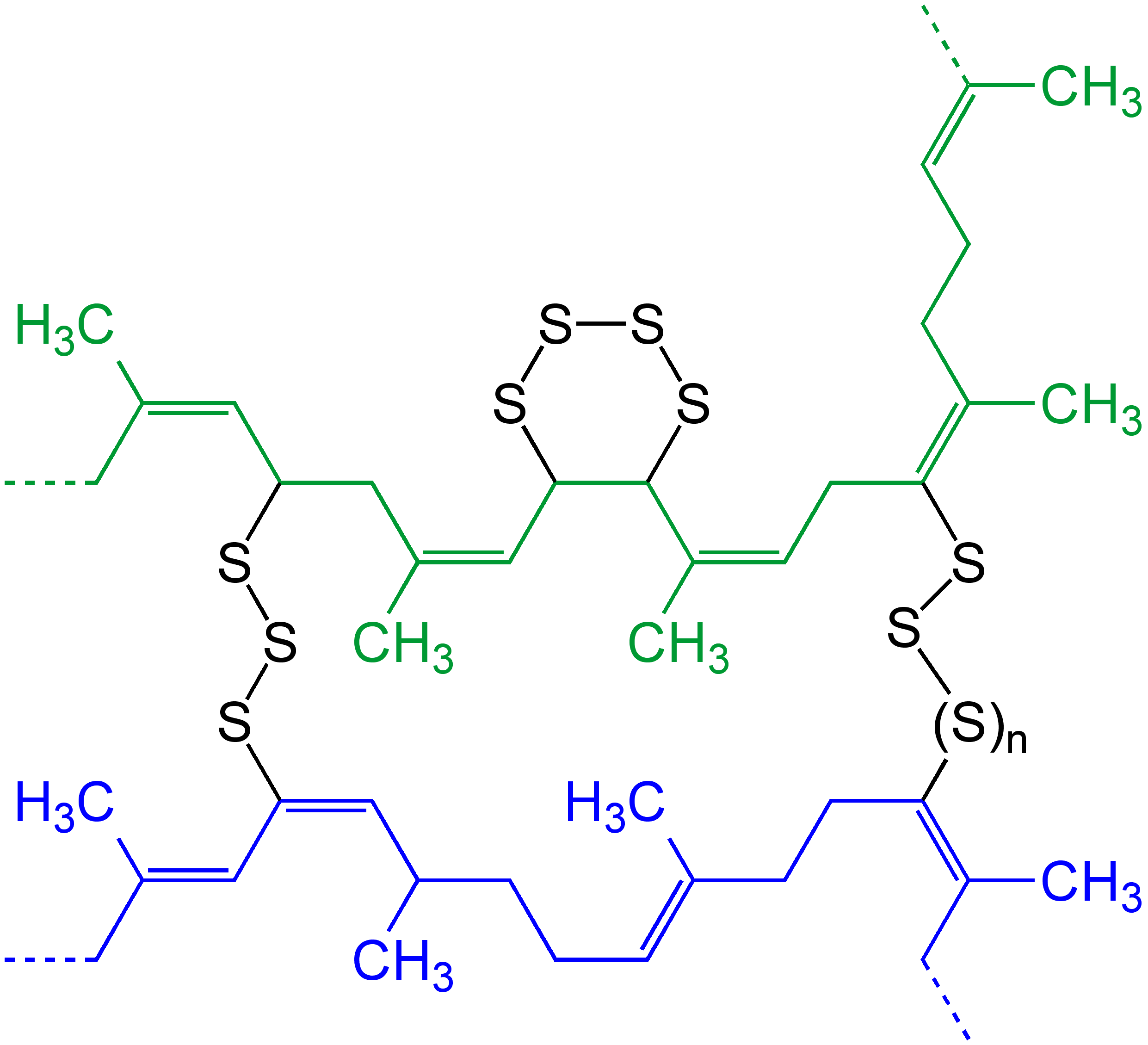 The details of vulcanization remain murky because the process converts mixtures of polymers to mixtures of insoluble derivatives. By design the reaction does not proceed to completion because fully crosslinked polymer would be too rigid for applications. There has long been uncertainly as to whether vulcanization proceeds in a radical or ionic manner.
It is agreed that the reactive sites, often referred to as 'cure sites', are the allyl groups (-CH=CH-CH2-). Sulfur forms bridge between these sites, crosslinking the polymer chains. These bridges may consist of one or several sulfur atoms and are separated by hundreds or thousands of carbons in the polymer chain.
Both the extent of crosslinking and the number of sulfur atoms in the crosslinks strongly influences the physical properties of the rubber produced:
* Excessive crosslinking can convert the rubber into a hard and brittle substance (i.e.
The details of vulcanization remain murky because the process converts mixtures of polymers to mixtures of insoluble derivatives. By design the reaction does not proceed to completion because fully crosslinked polymer would be too rigid for applications. There has long been uncertainly as to whether vulcanization proceeds in a radical or ionic manner.
It is agreed that the reactive sites, often referred to as 'cure sites', are the allyl groups (-CH=CH-CH2-). Sulfur forms bridge between these sites, crosslinking the polymer chains. These bridges may consist of one or several sulfur atoms and are separated by hundreds or thousands of carbons in the polymer chain.
Both the extent of crosslinking and the number of sulfur atoms in the crosslinks strongly influences the physical properties of the rubber produced:
* Excessive crosslinking can convert the rubber into a hard and brittle substance (i.e.
Benzo(d)thiazole-2-thiol_200.svg ,
Oxidative dimerization of MBT gives mercaptobenzothiazole
Thiuram.svg , Thiuram
Zn(Me2dtc)2Improved.png , Zinc bis(dimethyldithiocarbamate) (
Secondary accelerants have very fast vulcanization speeds with minimal induction time, making them unsuitable as primary accelerants in highly unsaturated rubbers such as NR or SBR. However, they can be used as primary accelerants in compounds with fewer curing site such as EPDM. Xanthates (principally, zinc isopropyl xanthate) are important in the vulcanization of latex, which is cured at relatively low temperature (100-120 °C), and therefore needs an inherently rapid accelerant. The major thiurams used are TMTD ( tetramethylthiuram disulfide) and TETD ( tetraethylthiuram disulfide). The major dithiocarbamates are the zinc salts ZDMC (
 Sulfur vulcanization is a
Sulfur vulcanization is a chemical process
In a scientific sense, a chemical process is a method or means of somehow changing one or more chemicals or chemical compounds. Such a chemical process can occur by itself or be caused by an outside force, and involves a chemical reaction of some ...
for converting natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and ...
or related polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s into materials of varying hardness, elasticity, and mechanical durability by heating them with sulfur or sulfur-containing compounds. Sulfur forms cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ing bridges between sections of polymer chain
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s which affects the mechanical and electronic properties. Many products are made with vulcanized rubber, including tire
A tire (American English) or tyre (British English) is a ring-shaped component that surrounds a wheel's rim to transfer a vehicle's load from the axle through the wheel to the ground and to provide traction on the surface over which t ...
s, shoe soles, hoses, and conveyor belts. The term is derived from Vulcan
Vulcan may refer to:
Mythology
* Vulcan (mythology), the god of fire, volcanoes, metalworking, and the forge in Roman mythology
Arts, entertainment and media Film and television
* Vulcan (''Star Trek''), name of a fictional race and their home p ...
, the Roman god
Roman mythology is the body of myths of ancient Rome as represented in the literature and visual arts of the Romans. One of a wide variety of genres of Roman folklore, ''Roman mythology'' may also refer to the modern study of these representat ...
of fire.
The main polymers subjected to sulfur vulcanization are polyisoprene
Polyisoprene is strictly speaking a collective name for polymers that are produced by polymerization of isoprene. In practice polyisoprene is commonly used to refer to synthetic ''cis''-1,4-polyisoprene, made by the industrial polymerisation of i ...
(natural rubber, NR), polybutadiene rubber (BR) and styrene-butadiene
Styrene-butadiene or styrene-butadiene rubber (SBR) describe families of synthetic rubbers derived from styrene and butadiene (the version developed by Goodyear is called Neolite). These materials have good abrasion resistance and good aging st ...
rubber (SBR), and ethylene propylene diene monomer rubber ( EPDM rubber). All of these materials contain alkene groups adjacent to methylene groups. Other specialty rubbers may also be vulcanized, such as nitrile rubber
Nitrile rubber, also known as nitrile butadiene rubber, NBR, Buna-N, and acrylonitrile butadiene rubber, is a synthetic rubber derived from acrylonitrile (ACN) and butadiene. Trade names include Perbunan, Nipol, Krynac and Europrene. This rubber i ...
(NBR) and butyl rubber
Butyl rubber, sometimes just called "butyl", is a synthetic rubber, a copolymer of isobutylene with isoprene. The abbreviation IIR stands for isobutylene isoprene rubber. Polyisobutylene, also known as "PIB" or polyisobutene, (C4H8)n, is the ho ...
(IIR). Vulcanization, in common with the curing of other thermosetting polymer
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening (" curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and ...
s, is generally irreversible. Efforts have focussed on developing de-vulcanization (see tire recycling
Tire recycling, or rubber recycling, is the process of recycling waste tires that are no longer suitable for use on vehicles due to wear or irreparable damage. These tires are a challenging source of waste, due to the large volume produced, th ...
) processes for recycling of rubber waste but with little success.
Structural and mechanistic details
 The details of vulcanization remain murky because the process converts mixtures of polymers to mixtures of insoluble derivatives. By design the reaction does not proceed to completion because fully crosslinked polymer would be too rigid for applications. There has long been uncertainly as to whether vulcanization proceeds in a radical or ionic manner.
It is agreed that the reactive sites, often referred to as 'cure sites', are the allyl groups (-CH=CH-CH2-). Sulfur forms bridge between these sites, crosslinking the polymer chains. These bridges may consist of one or several sulfur atoms and are separated by hundreds or thousands of carbons in the polymer chain.
Both the extent of crosslinking and the number of sulfur atoms in the crosslinks strongly influences the physical properties of the rubber produced:
* Excessive crosslinking can convert the rubber into a hard and brittle substance (i.e.
The details of vulcanization remain murky because the process converts mixtures of polymers to mixtures of insoluble derivatives. By design the reaction does not proceed to completion because fully crosslinked polymer would be too rigid for applications. There has long been uncertainly as to whether vulcanization proceeds in a radical or ionic manner.
It is agreed that the reactive sites, often referred to as 'cure sites', are the allyl groups (-CH=CH-CH2-). Sulfur forms bridge between these sites, crosslinking the polymer chains. These bridges may consist of one or several sulfur atoms and are separated by hundreds or thousands of carbons in the polymer chain.
Both the extent of crosslinking and the number of sulfur atoms in the crosslinks strongly influences the physical properties of the rubber produced:
* Excessive crosslinking can convert the rubber into a hard and brittle substance (i.e. ebonite
Ebonite is a brand name for a material generically known as hard rubber, and is obtained via vulcanizing natural rubber for prolonged periods. Ebonite may contain from 25% to 80% sulfur and linseed oil. Its name comes from its intended use as a ...
).
* Short crosslinks, possessing lower numbers of sulfur atoms, give the rubber better resistance to heat and weathering.
* Longer crosslinks, with higher numbers of sulfur atoms, give the rubber improved physical durability and tensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials t ...
.
Sulfur, by itself, is a slow vulcanizing agent and does not vulcanize synthetic polyolefins. Even with natural rubber, large amounts of sulfur as well as high temperatures and prolonged heating periods are necessary, with the end products often being of an unsatisfactory quality.
Since the early 1900s, various chemical additives have been developed to improve the speed and efficiency of vulcanization, as well as to control the nature of the cross-linking. When used together, this collection - the "cure package" - gives a rubber with particular properties.
Cure package
The cure package consists of various reagents that modify the kinetics and chemistry of crosslinking. These include accelerants, activators, retarders and inhibitors. Note that these are merely the additives used for vulcanization and that other compounds may also be added to the rubber, such asfillers
In processed animal foods, a filler is an ingredient added to provide dietary fiber, bulk or some other non-nutritive purpose.
Products like corncobs, feathers, soy, cottonseed hulls, peanut hulls, citrus pulp, screening, weeds, straw, and cere ...
, tackifier
Tackifiers are chemical compounds used in formulating adhesives to increase the tack, the stickiness of the surface of the adhesive. They are usually low-molecular weight compounds with high glass transition temperature. At low strain rate, they p ...
s, polymer stabilizers Polymer stabilizers (British: polymer stabilisers) are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation.
Common polymer degradation processes include oxidation, UV- ...
and antiozonant
An antiozonant, also known as anti-ozonant, is an organic compound that prevents or retards damage caused by ozone. The most important antiozonants are those which prevent degradation of elastomers like rubber. A number of research projects study ...
s.
Sulfur source
Ordinary sulfur (octasulfur, or S8) is rarely used, despite its low cost, because it is soluble in the polymer. High temperature vulcanisation with ordinary sulfur leads to rubber supersaturated with S8, upon cooling this migrates to the surface and crystallises as sulfur bloom. This can cause problems if multiple layers of rubber are being added to form a composite item, such as a tire. Instead, various forms of polymeric sulfur are used. It is also possible to replace sulfur with other sulfur-donating compounds, for example accelerants bearingdisulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
groups, in what is often termed "efficient vulcanization" (EV). Disulfur dichloride may also be used for "cold vulcanization".
Accelerants
Accelerant
Accelerants are substances that can bond, mix or disturb another substance and cause an increase in the speed of a natural, or artificial chemical process. Accelerants play a major role in chemistry—most chemical reactions can be hastened with an ...
s (accelerators) act much like catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
allowing vulcanization to be performed cooler yet faster and with a more efficient use of sulfur. They achieve this by reacting with and breaking the sulfur to form a reactive intermediate, referred to as a sulfurating agent. This, in turn, reacts with cure sites in the rubber to bring about vulcanization.
There are two major classes of vulcanization accelerants: primary accelerants and secondary accelerants (also known as ultra accelerants). Primary activators date from the use of ammonia in 1881, while secondary accelerants have been developed since around 1920.
;Primary (fast-accelerants)
Primary accelerants perform the bulk of the accelerating and mostly consist of thiazole
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular fo ...
s, often derivatised with sulfenamide groups. The principal compound is 2-mercaptobenzothiazole
2-Mercaptobenzothiazole is an organosulfur compound with the formula . It is used in the sulfur vulcanization of rubber.
Structure
The molecule is planar with a C=S double bond, so the name ''mercapto''benzothiazole is a misnomer. It is not a th ...
(MBT), which has been in use since the 1920s. It remains a moderately fast curing agent giving sulfur chains of a medium length, but its relatively short induction period
An induction period in chemical kinetics is an initial slow stage of a chemical reaction; after the induction period, the reaction accelerates. Ignoring induction periods can lead to runaway reactions.
In some catalytic reactions, a pre-catalyst n ...
can be a disadvantage. Other primary accelerants are essentially "masked" forms of MBT, which take time to decompose into MBT during vulcanization and thus have longer inductions periods.
Mercaptobenzothiazole
2-Mercaptobenzothiazole is an organosulfur compound with the formula . It is used in the sulfur vulcanization of rubber.
Structure
The molecule is planar with a C=S double bond, so the name ''mercapto''benzothiazole is a misnomer. It is not a th ...
(MBT)
Di(benzothiazool-2-yl)disulfide.svg , Mercaptobenzothiazole disulfide (MBTS)
N-Cyclohexylbenzothiazol-2-sulfenamide.svg, N-Cyclohexylbenzothiazol-2-sulfenamide (CBS)
N,N-dicyclohexyl-2-benzothiazolesulfenamide.svg , Dicyclohexyl-2-benzothiazolesulfenamide (DCBS)
disulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
(MBTS), and sulfenamide derivatives are produced by reacting this with primary amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
s like cyclohexylamine
Cyclohexylamine is an organic compound, belonging to the aliphatic amine class. It is a colorless liquid, although, like many amines, samples are often colored due to contaminants. It has a fishy odor and is miscible with water. Like other amines, ...
or tert-Butylamine. Secondary amines like dicyclohexylamine can be used and result in even slower accelerants. Such a slow accelerant is required in applications in which the rubber is being cured onto a metal component to which it is required to adhere, such as the steel cords in vehicle tires.
;Secondary (ultra-accelerants)
Secondary or ultra-accelerants are used in small amounts to augment the behaviour of primary accelerants. They act to boost the cure speed and increase cross-link density, but also shorten the induction time, which can lead to premature vulcanization. Chemically, they consist mainly of thio-carbonyl species such as thiurams, dithiocarbamate
In organic chemistry, a dithiocarbamate is a functional group with the general formula and structure . It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms (when only 1 oxygen is replaced the result is thioca ...
s, xanthate
150px, Sodium salt of ethyl xanthate
Xanthate usually refers to a salt with the formula (R = alkyl; M+ = Na+, K+), thus they are the metal-thioate/''O''-esters of dithiocarbonate. The name ''xanthates'' is derived from Ancient Greek ''xanthos' ...
s and organic thiourea
Thiourea () is an organosulfur compound with the formula and the structure . It is structurally similar to urea (), except that the oxygen atom is replaced by a sulfur atom (as implied by the ''thio-'' prefix); however, the properties of urea a ...
s; aromatic guanidine
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine predominantly in patients experie ...
s are also used. These compounds need to be combined with activators, typically zinc ions, in order to be fully active.
Ziram
Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications.
Applications
Known as zira ...
)
1,3-difenylguanidine t.png , diphenylguanidine (DPG)
zinc dimethyldithiocarbamate
Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications.
Applications
Known as zira ...
), ZDEC (zinc diethyldithiocarbamate) and ZDBC (zinc dibutyldithiocarbamate).
Activators
Activators consist of various metal salts, fatty acids, as well as nitrogen-containing bases, the most important these beingzinc oxide
Zinc oxide is an inorganic compound with the formula . It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cement ...
. Zinc actives many accelerants by coordination, for example causing thiuram to convert into ziram
Zinc dimethyldithiocarbamate is a coordination complex of zinc with dimethyldithiocarbamate. It is a pale yellow solid that is used as a fungicide, the sulfur vulcanization of rubber, and other industrial applications.
Applications
Known as zira ...
. Zinc also coordinates to the sulfur-chains of sulfurating agents, changing the most likely bond to break during cross-link formation. Ultimately, activators promote the efficient use of sulfur to give a high density of cross-links. Due to the low solubility of ZnO it is often combined with fatty acids such as stearic acid to form more soluble metallic soap, ''i.e.'', zinc stearate
Zinc stearate is a "zinc soap" that is widely used industrially. In this context, soap is used in its formal sense, a metal salt of a fatty acid: in this case stearic acid. It is a white solid that repels water. It is insoluble in polar solvents ...
.
Retarders and inhibitors
To ensure high quality vulcanization, the rubber, sulfur, accelerants, activators and other compounds are blended to give a homogeneous mixture. In practice, mixing can result in melting the sulfur (melting point 115 °C for S8). At these temperatures vulcanization can begin prematurely, which is often undesirable, as the mixture may still need to be pumped and moulded into its final form before it sets solid. Premature vulcanization is often called "scorch". Scorch can be prevented by the use of retarders or inhibitors, which increase theinduction period
An induction period in chemical kinetics is an initial slow stage of a chemical reaction; after the induction period, the reaction accelerates. Ignoring induction periods can lead to runaway reactions.
In some catalytic reactions, a pre-catalyst n ...
before vulcanization commences and thus provide scorch resistance. A retarder slows both the onset and rate of vulcanization, whereas inhibitors only delay the start of vulcanization and do not affect the rate to any great extent. In general inhibitors are preferred, with cyclohexylthiophthalimide (often termed PVI — pre-vulcanization inhibitor) being the most common example.
Devulcanization
The market for new raw rubber or equivalent is large. The auto industry consumes a substantial fraction of natural and synthetic rubber. Reclaimed rubber has altered properties and is unsuitable for use in many products, including tires. Tires and other vulcanized products are potentially amenable to devulcanization, but this technology has not produced material that can supplant unvulcanized materials. The main problem is that the carbon-sulfur linkages are not readily broken, without the input of costly reagents and heat. Thus, more than half of scrap rubber is simply burned for fuel.Inverse vulcanization
Although polymeric sulfur reverts to its monomer at room temperature, polymers consisting mostly of sulfur can be stabilized with organic linkers such as 1,3‐diisopropenylbenzene. This process is called inverse vulcanization and produces polymers where sulfur is the main component.History
The curing of rubber has been carried out since prehistoric times. The name of the first major civilization in Guatemala and Mexico, theOlmec
The Olmecs () were the earliest known major Mesoamerican civilization. Following a progressive development in Soconusco, they occupied the tropical lowlands of the modern-day Mexican states of Veracruz and Tabasco. It has been speculated that ...
, means 'rubber people' in the Aztec
The Aztecs () were a Mesoamerican culture that flourished in central Mexico in the post-classic period from 1300 to 1521. The Aztec people included different ethnic groups of central Mexico, particularly those groups who spoke the Nahuatl ...
language. Ancient Mesoamerica
Mesoamerica is a historical region and cultural area in southern North America and most of Central America. It extends from approximately central Mexico through Belize, Guatemala, El Salvador, Honduras, Nicaragua, and northern Costa Rica ...
ns, spanning from ancient Olmecs to Aztecs, extracted latex
Latex is an emulsion (stable dispersion) of polymer microparticles in water. Latexes are found in nature, but synthetic latexes are common as well.
In nature, latex is found as a milky fluid found in 10% of all flowering plants (angiosperms ...
from ''Castilla elastica
''Castilla elastica'', the Panama rubber tree, is a tree native to the tropical areas of Mexico, Central America, and northern South America. It was the principal source of latex among the Mesoamerican peoples in pre-Columbian times. The latex g ...
'', a type of rubber tree
''Hevea brasiliensis'', the Pará rubber tree, ''sharinga'' tree, seringueira, or most commonly, rubber tree or rubber plant, is a flowering plant belonging to the spurge family Euphorbiaceae originally native to the Amazon basin, but is now ...
in the area. The juice of a local vine, ''Ipomoea alba
''Ipomoea alba'', sometimes called the tropical white morning-glory or moonflower or moon vine, is a species of night-blooming morning glory, native to tropical and subtropical regions of North and South America, from Argentina to northern Mexic ...
'', was then mixed with this latex to create processed rubber as early as 1600 BCE. In the Western world, rubber remained a curiosity, although it was eventually used to produce waterproofed products, such as Mackintosh
The Mackintosh or raincoat (abbreviated as mac) is a form of waterproof raincoat, first sold in 1824, made of rubberised fabric.
The Mackintosh is named after its Scottish inventor Charles Macintosh, although many writers added a letter ''k' ...
rainwear, beginning in the early 1800s.
Modern developments
In 1832–1834Nathaniel Hayward
Nathaniel Manley Hayward (January 19, 1808 – July 18, 1865) was a US businessman and inventor best known for selling a patent to Charles Goodyear that Goodyear later used to develop the process of vulcanization
Biography
Nathaniel Haywar ...
and Friedrich Ludersdorf discovered that rubber treated with sulfur lost its stickiness. It is likely Hayward shared his discovery with Charles Goodyear
Charles Goodyear (December 29, 1800 – July 1, 1860) was an American self-taught chemist and manufacturing engineer who developed vulcanized rubber, for which he received patent number 3633 from the United States Patent Office on June 15, 1844. ...
, possibly inspiring him to make the discovery of vulcanization.
Thomas Hancock (1786–1865), a scientist and engineer, was the first to patent vulcanization of rubber. He was awarded a British patent on May 21, 1845. Three weeks later, on June 15, 1845, Charles Goodyear was awarded a patent in the United States. It was Hancock's friend William Brockedon
William Brockedon (13 October 1787 – 29 August 1854) was a 19th-century English painter, writer and inventor.
Early life
Brockedon was born at Totnes on 13 October 1787, son of a watchmaker. He was educated at a private school in Totnes, bu ...
who coined term 'vulcanization'.
Goodyear claimed that he had discovered vulcanization earlier, in 1839. He wrote the story of the discovery in 1853 in his autobiographical book ''Gum-Elastica''. Here is Goodyear's account of the invention, taken from ''Gum-Elastica''. Although the book is an autobiography, Goodyear chose to write it in the third person so that and referred to in the text are the author. He describes the scene in a rubber factory
A factory, manufacturing plant or a production plant is an industrial facility, often a complex consisting of several buildings filled with machinery, where workers manufacture items or operate machines which process each item into another. ...
where his brother worked:
The inventor made experiments to ascertain the effect of heat on the same compound that had decomposed in the mail-bags and other articles. He was surprised to find that the specimen, being carelessly brought into contact with a hot stove, charred like leather.Goodyear goes on to describe how his discovery was not readily accepted.
He directly inferred that if the process of charring could be stopped at the right point, it might divest the gum of its native adhesiveness throughout, which would make it better than the native gum. Upon further trial with heat, he was further convinced of the correctness of this inference, by finding that the India rubber could not be melted in boiling sulfur at any heat, but always charred. He made another trial of heating a similar fabric before an open fire. The same effect, that of charring the gum, followed. There were further indications of success in producing the desired result, as upon the edge of the charred portion appeared a line or border, that was not charred, but perfectly cured.Goodyear then goes on to describe how he moved to
Woburn, Massachusetts
Woburn ( ) is a city in Middlesex County, Massachusetts, United States. The population was 40,876 at the 2020 census. Woburn is located north of Boston. Woburn uses Massachusetts' mayor-council form of government, in which an elected mayor is ...
and carried out a series of systematic experiments to optimize the curing of rubber, collaborating with Nathaniel Hayward
Nathaniel Manley Hayward (January 19, 1808 – July 18, 1865) was a US businessman and inventor best known for selling a patent to Charles Goodyear that Goodyear later used to develop the process of vulcanization
Biography
Nathaniel Haywar ...
.
On ascertaining to a certainty that he had found the object of his search and much more, and that the new substance was proof against cold and the solvent of the native gum, he felt himself amply repaid for the past, and quite indifferent to the trials of the future.
Later developments
The discovery of the rubber-sulfur reaction revolutionized the use and applications of rubber, changing the face of the industrial world. Formerly, the only way to seal a small gap between moving machine parts was to useleather
Leather is a strong, flexible and durable material obtained from the tanning, or chemical treatment, of animal skins and hides to prevent decay. The most common leathers come from cattle, sheep, goats, equine animals, buffalo, pigs and hog ...
soaked in oil. This practice was acceptable only at moderate pressures, but above a certain point, machine designers were forced to compromise between the extra friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and material elements sliding against each other. There are several types of friction:
*Dry friction is a force that opposes the relative lateral motion of ...
generated by tighter packing and greater leakage of steam. Vulcanized rubber solved this problem. It could be formed to precise shapes and dimensions, it accepted moderate to large deformations under load and recovered quickly to its original dimensions once the load is removed. These exceptional qualities, combined with good durability and lack of stickiness, were critical for an effective sealing material. Further experiments in the processing and compounding of rubber by Hancock and his colleagues led to a more reliable process.
Around 1900, disulfiram
Disulfiram is a medication used to support the treatment of chronic alcoholism by producing an acute sensitivity to ethanol (drinking alcohol). Disulfiram works by inhibiting the enzyme acetaldehyde dehydrogenase, causing many of the effects of ...
was introduced as a vulcanizing agent, and became widely used.
In 1905 George Oenslager discovered that a derivative of aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starti ...
called thiocarbanilide accelerated the reaction of sulfur with rubber, leading to shorter cure times and reducing energy consumption. This breakthrough was almost as fundamental to the rubber industry as Goodyear's sulfur cure. Accelerators made the cure process faster, improved the reliability of the process and enabled vulcanization to be applied to synthetic polymers. One year after his discovery, Oenslager had found hundreds of applications for his additive. Thus, the science of accelerators and retarders was born. An accelerator speeds up the cure reaction, while a retarder delays it. A typical retarder is cyclohexylthiophthalimide. In the subsequent century chemists developed other accelerators and ultra-accelerators, which are used in the manufacture of most modern rubber goods.
See also
* Sulfur concreteReferences
{{Authority control Chemical processes Rubber 1837 introductions