Sulfonation on:
[Wikipedia]
[Google]
[Amazon]
Aromatic sulfonation is an
 Typical conditions involve heating the aromatic compound with sulfuric acid:
:C6H6 + H2SO4 → C6H5SO3H + H2O
Typical conditions involve heating the aromatic compound with sulfuric acid:
:C6H6 + H2SO4 → C6H5SO3H + H2O

 Sulfonation of
Sulfonation of
organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical ...
in which a hydrogen atom on an arene is replaced by a sulfonic acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is k ...
functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the r ...
in an electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic n ...
. Aryl sulfonic acids are used as detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are m ...
s, dye
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and ...
, and drug
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via inhala ...
s.
Stoichiometry and mechanism
Sulfur trioxide
Sulfur trioxide (alternative spelling sulphur trioxide, also known as ''nisso sulfan'') is the chemical compound with the formula SO3. It has been described as "unquestionably the most important economically" sulfur oxide. It is prepared on an ind ...
or its protonated derivative is the actual electrophile in this electrophilic aromatic substitution.
To drive the equilibrium, dehydrating agents such as thionyl chloride can be added.
:C6H6 + H2SO4 + SOCl2 → C6H5SO3H + SO2 + 2 HCl
Chlorosulfuric acid
Chlorosulfuric acid (IUPAC name: sulfurochloridic acid) is the inorganic compound with the formula HSO3Cl. It is also known as chlorosulfonic acid, being the sulfonic acid of chlorine. It is a distillable, colorless liquid which is hygroscopic an ...
is also an effective agent:
:C6H6 + HSO3Cl → C6H5SO3H + HCl
In contrast to aromatic nitration
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols an ...
and most other electrophilic aromatic substitutions this reaction is reversible. Sulfonation takes place in concentrated acidic conditions and desulfonation is the mode of action in a dilute hot aqueous acid. The reaction is very useful in protecting the aromatic system because of this reversibility. Due to their electron withdrawing effects, sulfonate protecting groups can be used to prevent electrophilic aromatic substitution. They can also be installed as directing groups to affect the position where a substitution may take place.T.W> Graham Solomons: ''Organic Chemistry'', 11th Edition, Wiley, Hoboken, NJ, 2013, p. 676, .
Specialized sulfonation methods
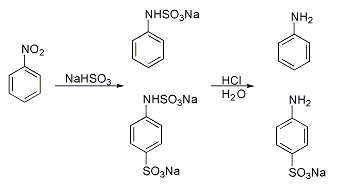
Many method have been developed for introducing sulfonate groups aside from direction sulfonation.Piria reaction
A classic named reaction is the Piria reaction (Raffaele Piria
Raffaele Piria ( Scilla 20 August 1814 – Turin 18 July 1865) was an Italian chemist from Scilla, who lived in Palmi. He converted the substance Salicin into a sugar and a second component, which on oxidation becomes salicylic acid, a major c ...
, 1851) in which nitrobenzene
Nitrobenzene is an organic compound with the chemical formula Phenyl, C6H5Nitro compound, NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from be ...
is reacted with a metal bisulfite
The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion . Salts containing the ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na2S2O5). Sodium metabisulfite diss ...
forming an aminosulfonic acid as a result of combined nitro group reduction and sulfonation.

Tyrer sulfonation process
In the Tyrer sulfonation process (1917), at some time of technological importance, benzene vapor is led through a vessel containing 90% sulfuric acid the temperature of which is increased from 100 to 180°C. Water and benzene are continuously removed in a condenser and the benzene layer fed back to the vessel. In this way an 80% yield is obtained.Applications
Aromatic sulfonic acids are intermediates in the preparation ofdye
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and ...
s and many pharmaceuticals. Sulfonation of aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starti ...
s lead to a large group of sulfa drug
Sulfonamide is a functional group (a part of a molecule) that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimi ...
s.
polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the Aromatic hydrocarbon, aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin pe ...
is used to make sodium polystyrene sulfonate
Polystyrene sulfonates are a group of medications used to treat high blood potassium. Effects generally take hours to days. They are also used to remove potassium, calcium, and sodium from solutions in technical applications.
Common side effect ...
, a common ion exchange resin
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange. It is an insoluble matrix (or support structure) normally in the form of small (0.25–1.43 mm radius) microbeads, usually white or ...
for water softening
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also exten ...
.
Reactions of aryl sulfonic acids
As afunctional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the r ...
, aryl sulfonic acids undergo desulfonation when heated in water:
:RC6H4SO3H + H2O → RC6H5 + H2SO4
When treated with strong base, benzenesulfonic acid derivatives convert to phenols.
:C6H5SO3H + 2 NaOH → C6H5OH + Na2SO4 + H2O
See also
* Electrophilic halogenation * Nitration * PerchlorylbenzeneReferences
{{DEFAULTSORT:Aromatic Sulfonation Substitution reactions