Spin isomers of hydrogen on:
[Wikipedia]
[Google]
[Amazon]


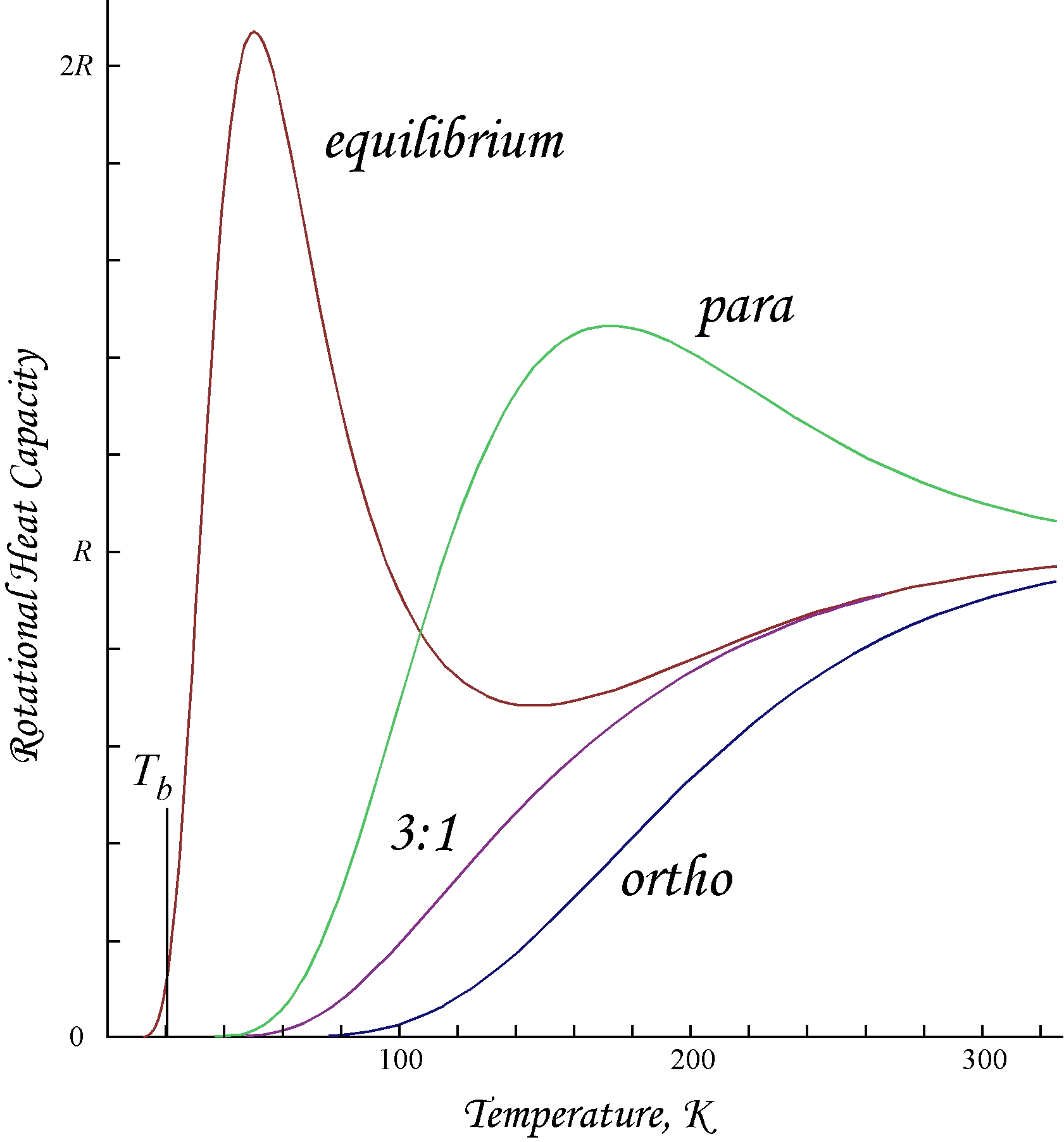 Because of the antisymmetry-imposed restriction on possible rotational states, orthohydrogen has residual rotational energy at low temperature wherein nearly all the molecules are in the ''J'' = 1 state (molecules in the symmetric spin-triplet state cannot fall into the lowest, symmetric rotational state) and possesses nuclear-spin
Because of the antisymmetry-imposed restriction on possible rotational states, orthohydrogen has residual rotational energy at low temperature wherein nearly all the molecules are in the ''J'' = 1 state (molecules in the symmetric spin-triplet state cannot fall into the lowest, symmetric rotational state) and possesses nuclear-spin
accessed 10 May 2015. * * * * * {{cite journal , author = Karl Friedrich Bonhoeffer, Bonhoeffer KF, Harteck P , title = Para- and ortho hydrogen , journal = Zeitschrift für Physikalische Chemie B , volume = 4 , issue = 1–2 , pages = 113–141 , year = 1929 * Oxford Instruments, Date Unknown, "Boosting the Sensitivity of NMR Spectroscopy using Parahydrogen" Hydrogen physics Hydrogen technologies Hydrogen

Molecular hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
occurs in two isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
I ...
ic forms, one with its two proton nuclear spin
In atomic physics, the spin quantum number is a quantum number (designated ) which describes the intrinsic angular momentum (or spin angular momentum, or simply spin) of an electron or other particle. The phrase was originally used to describe t ...
s aligned parallel (orthohydrogen), the other with its two proton spins aligned antiparallel (parahydrogen).P. Atkins and J. de Paula, Atkins' ''Physical Chemistry'', 8th edition (W.H.Freeman 2006), p. 451–2 These two forms are often referred to as spin isomers or as nuclear spin isomers.
Parahydrogen is in a lower energy state than is orthohydrogen. At room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
and thermal equilibrium
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in ...
, thermal excitation causes hydrogen to consist of approximately 75% orthohydrogen and 25% parahydrogen. When hydrogen is liquified at low temperature, there is a slow spontaneous transition to a predominantly para ratio, with the released energy having implications for storage. Essentially pure parahydrogen form can be obtained at very low temperatures, but it is not possible to obtain a sample containing more than 75% orthohydrogen by heating.
A mixture or 50:50 mixture of ortho- and parahydrogen can be made in the laboratory by passing it over an iron(III) oxide catalyst at liquid nitrogen
Liquid nitrogen—LN2—is nitrogen in a liquid state at low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, low viscosity liquid that is wide ...
temperature (77 K) or by storing hydrogen at 77 K for 2–3 hours in the presence of activated charcoal. In the absence of a catalyst, gas phase parahydrogen takes days to relax to normal hydrogen at room temperature while it takes hours to do so in organic solvents.
Nuclear spin states of H2
Eachhydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxi ...
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
() consists of two hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen consti ...
s linked by a covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
. If we neglect the small proportion of deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one n ...
and tritium
Tritium ( or , ) or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with half-life about 12 years. The nucleus of tritium (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of ...
which may be present, each hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen consti ...
consists of one proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
and one electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kno ...
. Each proton has an associated magnetic moment
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagnets ...
, which is associated with the proton's spin of . In the molecule, the spins of the two hydrogen nuclei (protons) couple to form a triplet state
In quantum mechanics, a triplet is a quantum state of a system with a spin of quantum number =1, such that there are three allowed values of the spin component, = −1, 0, and +1.
Spin, in the context of quantum mechanics, is not a mechanical ...
known as orthohydrogen, and a singlet state
In quantum mechanics, a singlet state usually refers to a system in which all electrons are paired. The term 'singlet' originally meant a linked set of particles whose net angular momentum is zero, that is, whose overall spin quantum number s=0. A ...
known as parahydrogen.
The triplet orthohydrogen state has total nuclear spin ''I'' = 1 so that the component along a defined axis can have the three values ''M''''I'' = 1, 0, or −1. The corresponding nuclear spin wavefunctions are , and . This formalism uses standard bra–ket notation
In quantum mechanics, bra–ket notation, or Dirac notation, is used ubiquitously to denote quantum states. The notation uses angle brackets, and , and a vertical bar , to construct "bras" and "kets".
A ket is of the form , v \rangle. Mathemat ...
; the symbol ↑ represents the spin-up wavefunction
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements ma ...
and the symbol ↓ the spin-down wavefunction for a nucleus, so ↑↓ means that the first nucleus is up and the second down. Each orthohydrogen energy level then has a (nuclear) spin degeneracy of three, meaning that it corresponds to three states of the same energy (in the absence of a magnetic field). The singlet parahydrogen state has nuclear spin quantum numbers ''I'' = 0 and ''M''''I'' = 0, with wavefunction . Since there is only one possibility, each parahydrogen level has a spin degeneracy of one and is said to be non-degenerate.
Allowed rotational energy levels
Since protons have spin , they arefermion
In particle physics, a fermion is a particle that follows Fermi–Dirac statistics. Generally, it has a half-odd-integer spin: spin , spin , etc. In addition, these particles obey the Pauli exclusion principle. Fermions include all quarks and ...
s and the permutational antisymmetry of the total wavefunction imposes restrictions on the possible rotational states of the two forms of . Orthohydrogen, with symmetric nuclear spin functions, can only have rotational wavefunctions that are antisymmetric with respect to permutation of the two protons, corresponding to odd values of the rotational quantum number ''J''; conversely, parahydrogen with an antisymmetric nuclear spin function, can only have rotational wavefunctions that are symmetric with respect to permutation of the two protons, corresponding to even ''J''.
The para form whose lowest level is ''J'' = 0 is more stable by 1.455 kJ/mol than the ortho form whose lowest level is ''J'' = 1. The ratio between numbers of ortho and para molecules is about 3:1 at standard temperature where many rotational energy levels are populated, favoring the ortho form as a result of thermal energy. However, at low temperatures only the ''J'' = 0 level is appreciably populated, so that the para form dominates at low temperatures (approximately 99.8% at 20 K). The heat of vaporization
The enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. T ...
is only 0.904 kJ/mol. As a result, ortho liquid hydrogen equilibrating to the para form releases enough energy to cause significant loss by boiling.
Thermal properties
Applying therigid rotor
In rotordynamics, the rigid rotor is a mechanical model of rotating systems. An arbitrary rigid rotor is a 3-dimensional rigid object, such as a top. To orient such an object in space requires three angles, known as Euler angles. A special rigi ...
approximation, the energies and degeneracies of the rotational states are given by:
: .
The rotational partition function is conventionally written as:
: .
However, as long as the two spin isomers are not in equilibrium, it is more useful to write separate partition functions for each:
:
The factor of 3 in the partition function for orthohydrogen accounts for the spin degeneracy associated with the +1 spin state; when equilibrium between the spin isomers is possible, then a general partition function incorporating this degeneracy difference can be written as:
:
The molar rotational energies and heat capacities are derived for any of these cases from:
:
Plots shown here are molar rotational energies and heat capacities for ortho- and parahydrogen, and the "normal" ortho:para ratio (3:1) and equilibrium mixtures:

 Because of the antisymmetry-imposed restriction on possible rotational states, orthohydrogen has residual rotational energy at low temperature wherein nearly all the molecules are in the ''J'' = 1 state (molecules in the symmetric spin-triplet state cannot fall into the lowest, symmetric rotational state) and possesses nuclear-spin
Because of the antisymmetry-imposed restriction on possible rotational states, orthohydrogen has residual rotational energy at low temperature wherein nearly all the molecules are in the ''J'' = 1 state (molecules in the symmetric spin-triplet state cannot fall into the lowest, symmetric rotational state) and possesses nuclear-spin entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
due to the triplet state's threefold degeneracy. The residual energy is significant because the rotational energy levels are relatively widely spaced in ; the gap between the first two levels when expressed in temperature units is twice the characteristic rotational temperature The characteristic rotational temperature ( or ) is commonly used in statistical thermodynamics to simplify the expression of the rotational partition function and the rotational contribution to molecular thermodynamic properties. It has units of ...
for :
: .
This is the ''T'' = 0 intercept seen in the molar energy of orthohydrogen. Since "normal" room-temperature hydrogen is a 3:1 ortho:para mixture, its molar residual rotational energy at low temperature is (3/4) × 2''Rθ''rot ≈ 1091 J/mol, which is somewhat larger than the enthalpy of vaporization
The enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. T ...
of normal hydrogen, 904 J/mol at the boiling point, ''T''b ≈ 20.369 K. Notably, the boiling points of parahydrogen and normal (3:1) hydrogen are nearly equal; for parahydrogen ∆Hvap ≈ 898 J/mol at ''T''b ≈ 20.277 K, and it follows that nearly all the residual rotational energy of orthohydrogen is retained in the liquid state.
However, orthohydrogen is thermodynamically unstable at low temperatures and spontaneously converts into parahydrogen. This process lacks any natural de-excitation radiation mode, so it is slow in the absence of a catalyst which can facilitate interconversion of the singlet and triplet spin states. At room temperature, hydrogen contains 75% orthohydrogen, a proportion which the liquefaction process preserves if carried out in the absence of a catalyst
Catalysis () is the process of increasing the reaction rate, rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the ...
like ferric oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally ...
, activated carbon
Activated carbon, also called activated charcoal, is a form of carbon commonly used to filter contaminants from water and air, among many other uses. It is processed (activated) to have small, low-volume pores that increase the surface area avail ...
, platinized asbestos, rare earth metals, uranium compounds, chromic oxide, or some nickel compounds to accelerate the conversion of the liquid hydrogen
Liquid hydrogen (LH2 or LH2) is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form.
To exist as a liquid, H2 must be cooled below its critical point of 33 K. However, for it to be in a fully liq ...
into parahydrogen. Alternatively, additional refrigeration equipment can be used to slowly absorb the heat that the orthohydrogen fraction will (more slowly) release as it spontaneously converts into parahydrogen. If orthohydrogen is not removed from rapidly liquified hydrogen, without a catalyst, the heat released during its decay can boil off as much as 50% of the original liquid.
History
The unusual heat capacity of hydrogen was discovered in 1912 byArnold Eucken
Arnold Thomas Eucken (3 July 1884 – 16 June 1950) was a German chemist and physicist. He examined the energy states of the Hydrogen atom and contributed to knowledge of the atomic structure. He also contributed to chemical engineering and proce ...
. The two forms of molecular hydrogen were first proposed by Werner Heisenberg
Werner Karl Heisenberg () (5 December 1901 – 1 February 1976) was a German theoretical physicist and one of the main pioneers of the theory of quantum mechanics. He published his work in 1925 in a Über quantentheoretische Umdeutung kinematis ...
and Friedrich Hund
Friedrich Hermann Hund (4 February 1896 – 31 March 1997) was a German physicist from Karlsruhe known for his work on atoms and molecules.
Scientific career
Hund worked at the Universities of Rostock, Leipzig, Jena, Frankfurt am Main, and Göt ...
in 1927. Taking into account this theoretical framework, pure parahydrogen was first synthesized by Paul Harteck
Paul Karl Maria Harteck (20 July 190222 January 1985) was an Austrian physical chemist. In 1945 under Operation Epsilon in "the big sweep" throughout Germany, Harteck was arrested by the allied British and American Armed Forces for suspicion o ...
and Karl Friedrich Bonhoeffer
Karl-Friedrich Bonhoeffer (13 January 1899 – 15 May 1957) was a German chemist.
Education and career
Born in Breslau, he was an older brother of martyred theologian Dietrich Bonhoeffer. His father was neurologist Karl Bonhoeffer and his mot ...
in 1929 at the Kaiser Wilhelm Institute for Physical Chemistry and Electrochemistry
The Fritz Haber Institute of the Max Planck Society (FHI) is a science research institute located at the heart of the academic district of Dahlem, in Berlin, Germany.
The original Kaiser Wilhelm Institute for Physical Chemistry and Electrochem ...
. When Heisenberg was awarded the 1932 Nobel prize in physics for the creation of quantum mechanics, this discovery of the "allotropic forms of hydrogen" was singled out as its most noteworthy application. Further work on the properties and chemical reactivity of parahydrogen was carried out in the following decade by Elly Agallidis
Elly Schwab-Agallidis (born Elly Agallidis, el, Έλλη Αγαλλίδου, ; – ) was a Greek physicist/physical chemist and one of the first women in Greece to be awarded a PhD in the field. She was the wife of Georg-Maria Schwab, who met ...
and Georg-Maria Schwab
Georg-Maria Schwab (, el, Γεώργιος Σβαμπ; 3 February 1899 – 23 December 1984) was a German-Greek physical chemist recognised for his important contributions in the field of catalysis and the kinetics thereof.
Schwab's early aca ...
.
Modern isolation of pure parahydrogen has since been achieved using rapid in-vacuum deposition of millimeters thick solid parahydrogen (p–) samples which are notable for their excellent optical qualities.
Use in NMR and MRI
When an excess of parahydrogen is used duringhydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic c ...
reactions (instead of the normal mixture of orthohydrogen to parahydrogen of 3:1), the resultant product exhibits hyperpolarized signals in proton NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
spectra, an effect termed PHIP (Parahydrogen Induced Polarisation) or, equivalently, PASADENA (Parahydrogen And Synthesis Allow Dramatically Enhanced Nuclear Alignment; named for first recognition of the effect by Bowers and Weitekamp of Caltech
The California Institute of Technology (branded as Caltech or CIT)The university itself only spells its short form as "Caltech"; the institution considers other spellings such a"Cal Tech" and "CalTech" incorrect. The institute is also occasional ...
), a phenomenon that has been used to study the mechanism of hydrogenation reactions.
Signal amplification by reversible exchange (SABRE) is a technique to hyperpolarize samples without chemically modifying them. Compared to orthohydrogen or organic molecules, a much greater fraction of the hydrogen nuclei in parahydrogen align with an applied magnetic field. In SABRE, a metal center reversibly binds to both the test molecule and a parahydrogen molecule facilitating the target molecule to pick up the polarization of the parahydrogen. This technique can be improved and utilized for a wide range of organic molecules by using an intermediate "relay" molecule like ammonia. The ammonia efficiently binds to the metal center and picks up the polarization from the parahydrogen. The ammonia then transfers it other molecules that don't bind as well to the metal catalyst. This enhanced NMR signal allows the rapid analysis of very small amounts of material and has great potential for applications in magnetic resonance imaging
Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomy and the physiological processes of the body. MRI scanners use strong magnetic fields, magnetic field gradients, and radio waves ...
.
Deuterium
Diatomicdeuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one n ...
() has nuclear spin isomers like diatomic hydrogen, but with different populations of the two forms because the deuterium nucleus (deuteron) is a boson
In particle physics, a boson ( ) is a subatomic particle whose spin quantum number has an integer value (0,1,2 ...). Bosons form one of the two fundamental classes of subatomic particle, the other being fermions, which have odd half-integer s ...
with nuclear spin equal to one. There are six possible nuclear spin wave functions which are ortho or symmetric to exchange of the two nuclei, and three which are para or antisymmetric. Ortho states correspond to even rotational levels with symmetric rotational functions so that the total wavefunction is symmetric as required for the exchange of two bosons, and para states correspond to odd rotational levels. The ground state (''J'' = 0) populated at low temperature is ortho, and at standard temperature the ortho:para ratio is 2:1.
Other substances with spin isomers
Other molecules and functional groups containing two hydrogen atoms, such aswater
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
and methylene (CH2), also have ortho- and para- forms (e.g. orthowater and parawater), but this is of little significance for their thermal properties. Their ortho:para ratios differ from that of dihydrogen. The ortho and para forms of water have recently been isolated. Para water was found to be 25% more reactive for a proton-transfer reaction.
Molecular oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well ...
() also exists in three lower-energy triplet states and one singlet state, as ground-state paramagnetic triplet oxygen
Triplet oxygen, 3O2, refers to the ''S'' = 1 electronic ground state of molecular oxygen (dioxygen). It is the most stable and common allotrope of oxygen. Molecules of triplet oxygen contain two unpaired electrons, making triplet oxygen an unus ...
and energized highly reactive diamagnetic singlet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambie ...
. These states arise from the spins of their unpaired electron
In chemistry, an unpaired electron is an electron that occupies an orbital of an atom singly, rather than as part of an electron pair. Each atomic orbital of an atom (specified by the three quantum numbers n, l and m) has a capacity to contain ...
s, not their protons or nuclei.
References
Further reading
* Aline Léon, Ed. 2008, ''Hydrogen Technology: Mobile and Portable Applications,'' pp. 93–101, New York, NY:Springer Science & Business, , seaccessed 10 May 2015. * * * * * {{cite journal , author = Karl Friedrich Bonhoeffer, Bonhoeffer KF, Harteck P , title = Para- and ortho hydrogen , journal = Zeitschrift für Physikalische Chemie B , volume = 4 , issue = 1–2 , pages = 113–141 , year = 1929 * Oxford Instruments, Date Unknown, "Boosting the Sensitivity of NMR Spectroscopy using Parahydrogen" Hydrogen physics Hydrogen technologies Hydrogen