securin on:
[Wikipedia]
[Google]
[Amazon]
Securin is a protein involved in control of the metaphase-anaphase transition and anaphase onset. Following bi-orientation of chromosome pairs and inactivation of the spindle checkpoint system, the underlying regulatory system, which includes securin, produces an abrupt stimulus that induces highly synchronous chromosome separation in
 Securin is initially present in the cytoplasm and binds to
Securin is initially present in the cytoplasm and binds to
 Securin has 5 known phosphorylation sites that are targets of Cdk1; 2 sites at the N-terminal in the Ken-Box and D-box region are known to affect APC recognition and ubiquitination (Figure 2). To initiate the onset of anaphase, securin is dephosphorylated by
Securin has 5 known phosphorylation sites that are targets of Cdk1; 2 sites at the N-terminal in the Ken-Box and D-box region are known to affect APC recognition and ubiquitination (Figure 2). To initiate the onset of anaphase, securin is dephosphorylated by
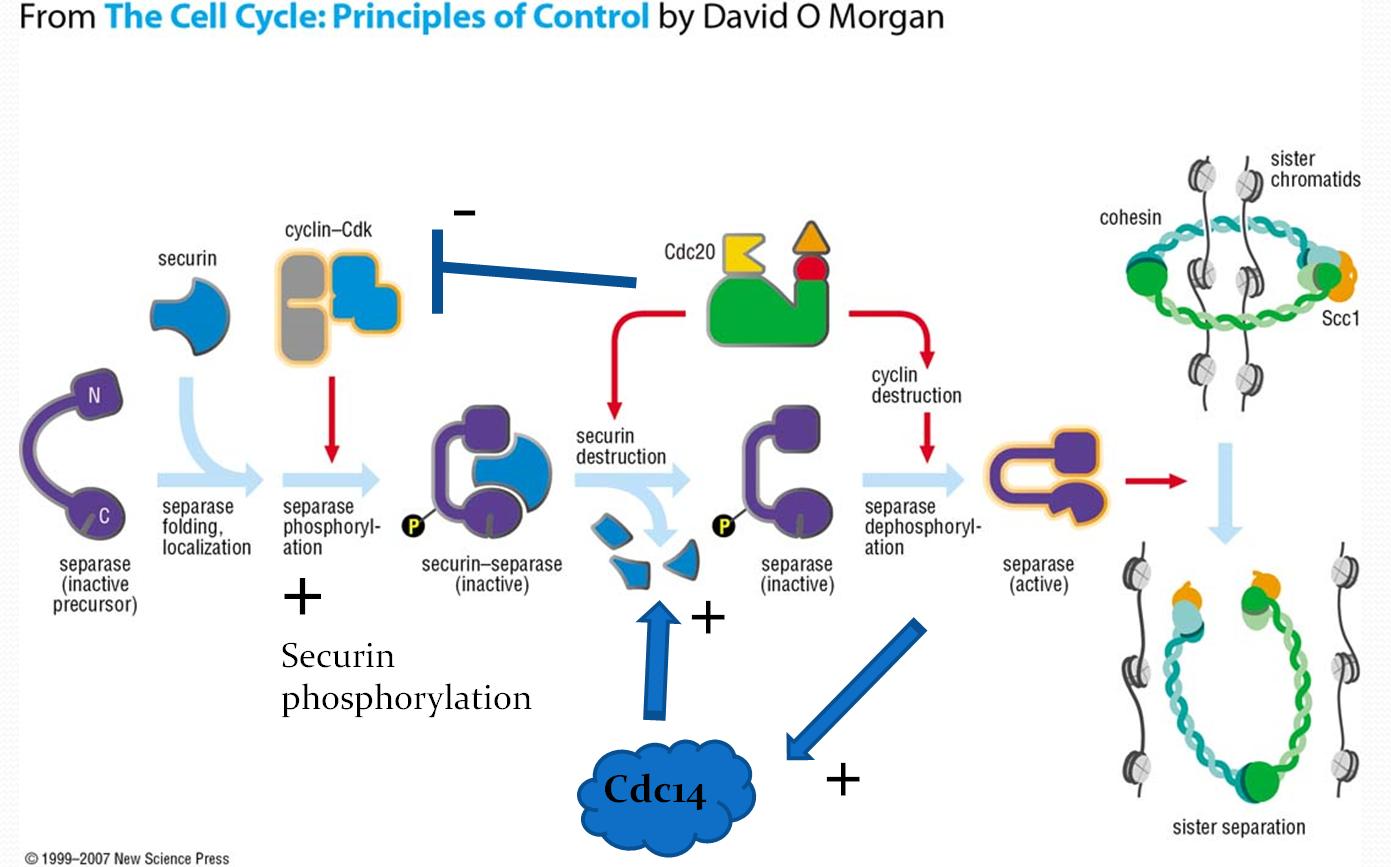
 It is thought that securin integrates multiple regulatory inputs to make separase activation switch-like, resulting in sudden, coordinated anaphase. This likely involves a network with several feedback loops, including positive feedback which leads to switch-like behavior. One proposed signaling pathway generating switch-like behavior contains a positive feedback loop for activation of Cdc14 by separase, leading to dephosphorylation and degradation of securin (Figure 3).
David Morgan’s group found that segregation time of chromosomes 4 and 5 is significantly elongated in budding-yeast strains with mutations in the 2 N-terminal securin phosphorylation sites and securin deletion strains. In addition, these mutant strains exhibited very high rates of mis-segregation compared to normal behavior. Switch-like characteristics are necessary to trigger quick, coordinated chromosomal segregation in anaphase. This means that strong inactivation of separase by securin followed by sudden, rapid destruction of securin and activation of separase is vital for proper anaphase.
Overall, securin and separase act in an anaphase-regulating network. Figure 4 depicts a potential network diagram.
It is thought that securin integrates multiple regulatory inputs to make separase activation switch-like, resulting in sudden, coordinated anaphase. This likely involves a network with several feedback loops, including positive feedback which leads to switch-like behavior. One proposed signaling pathway generating switch-like behavior contains a positive feedback loop for activation of Cdc14 by separase, leading to dephosphorylation and degradation of securin (Figure 3).
David Morgan’s group found that segregation time of chromosomes 4 and 5 is significantly elongated in budding-yeast strains with mutations in the 2 N-terminal securin phosphorylation sites and securin deletion strains. In addition, these mutant strains exhibited very high rates of mis-segregation compared to normal behavior. Switch-like characteristics are necessary to trigger quick, coordinated chromosomal segregation in anaphase. This means that strong inactivation of separase by securin followed by sudden, rapid destruction of securin and activation of separase is vital for proper anaphase.
Overall, securin and separase act in an anaphase-regulating network. Figure 4 depicts a potential network diagram.
Controlling the Cell Cycle: Anaphase Onset
Mitosis Cell biology
anaphase
Anaphase () is the stage of mitosis after the process of metaphase, when replicated chromosomes are split and the newly-copied chromosomes (daughter chromatids) are moved to opposite poles of the cell. Chromosomes also reach their overall max ...
.
Securin and Separase
 Securin is initially present in the cytoplasm and binds to
Securin is initially present in the cytoplasm and binds to separase
Separase, also known as separin, is a cysteine protease responsible for triggering anaphase by hydrolysing cohesin, which is the protein responsible for binding sister chromatids during the early stage of anaphase. In humans, separin is encod ...
, a protease that degrades the cohesin rings that link the two sister chromatids. Separase is vital for onset of anaphase. This securin-separase complex is maintained when securin is phosphorylated by Cdk1
Cyclin-dependent kinase 1 also known as CDK1 or cell division cycle protein 2 homolog is a highly conserved protein that functions as a serine/threonine protein kinase, and is a key player in cell cycle regulation. It has been highly studied in th ...
, inhibiting ubiquitination. When bound to securin, separase is not functional.
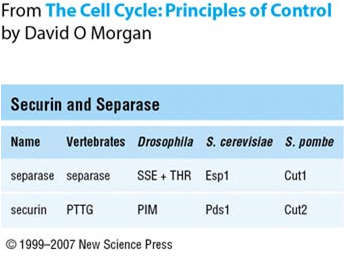
In addition, both securin and separase are well-conserved proteins (Figure 1). Note that separase cannot function without initially forming the securin-separase complex. This is because securin helps properly fold separase into the functional conformation. However, yeast does not appear to require securin to form functional separase as anaphase occurs in yeast with a securin deletion mutation.
Role of Securin in the onset of Anaphase
Basic mechanism
 Securin has 5 known phosphorylation sites that are targets of Cdk1; 2 sites at the N-terminal in the Ken-Box and D-box region are known to affect APC recognition and ubiquitination (Figure 2). To initiate the onset of anaphase, securin is dephosphorylated by
Securin has 5 known phosphorylation sites that are targets of Cdk1; 2 sites at the N-terminal in the Ken-Box and D-box region are known to affect APC recognition and ubiquitination (Figure 2). To initiate the onset of anaphase, securin is dephosphorylated by Cdc14 ''Cdc14'' and Cdc14 are a gene and its protein product respectively. Cdc14 is found in most of the eukaryotes. Cdc14 was defined by Hartwell in his famous screen for loci that control the cell cycle of Saccharomyces cerevisiae. Cdc14 was later sho ...
and other phosphatases. Dephosphorylated securin is recognized by the Anaphase-Promoting Complex (APC) bound primarily to Cdc20
The cell division cycle protein 20 homolog is an essential regulator of cell division that is encoded by the ''CDC20'' gene in humans. To the best of current knowledge its most important function is to activate the anaphase promoting complex (APC/ ...
(Cdh1 is also an activating substrate of APC). The APCCdc20 complex ubiquitinates securin and targets it for degradation by 26S proteasome. This results in free separase that is able to destroy cohesin and initiate chromosome separation.
Network characteristics
 It is thought that securin integrates multiple regulatory inputs to make separase activation switch-like, resulting in sudden, coordinated anaphase. This likely involves a network with several feedback loops, including positive feedback which leads to switch-like behavior. One proposed signaling pathway generating switch-like behavior contains a positive feedback loop for activation of Cdc14 by separase, leading to dephosphorylation and degradation of securin (Figure 3).
David Morgan’s group found that segregation time of chromosomes 4 and 5 is significantly elongated in budding-yeast strains with mutations in the 2 N-terminal securin phosphorylation sites and securin deletion strains. In addition, these mutant strains exhibited very high rates of mis-segregation compared to normal behavior. Switch-like characteristics are necessary to trigger quick, coordinated chromosomal segregation in anaphase. This means that strong inactivation of separase by securin followed by sudden, rapid destruction of securin and activation of separase is vital for proper anaphase.
Overall, securin and separase act in an anaphase-regulating network. Figure 4 depicts a potential network diagram.
It is thought that securin integrates multiple regulatory inputs to make separase activation switch-like, resulting in sudden, coordinated anaphase. This likely involves a network with several feedback loops, including positive feedback which leads to switch-like behavior. One proposed signaling pathway generating switch-like behavior contains a positive feedback loop for activation of Cdc14 by separase, leading to dephosphorylation and degradation of securin (Figure 3).
David Morgan’s group found that segregation time of chromosomes 4 and 5 is significantly elongated in budding-yeast strains with mutations in the 2 N-terminal securin phosphorylation sites and securin deletion strains. In addition, these mutant strains exhibited very high rates of mis-segregation compared to normal behavior. Switch-like characteristics are necessary to trigger quick, coordinated chromosomal segregation in anaphase. This means that strong inactivation of separase by securin followed by sudden, rapid destruction of securin and activation of separase is vital for proper anaphase.
Overall, securin and separase act in an anaphase-regulating network. Figure 4 depicts a potential network diagram.

References
{{reflistExternal links
* Video by David Morgan explaining action of securin and separin (in MP4 format): http://media.hhmi.org/ibio/morgan/morgan_3.mp4 * and in other formats:Controlling the Cell Cycle: Anaphase Onset
Mitosis Cell biology