Seawater on:
[Wikipedia]
[Google]
[Amazon]


 Seawater, or salt water, is
Seawater, or salt water, is
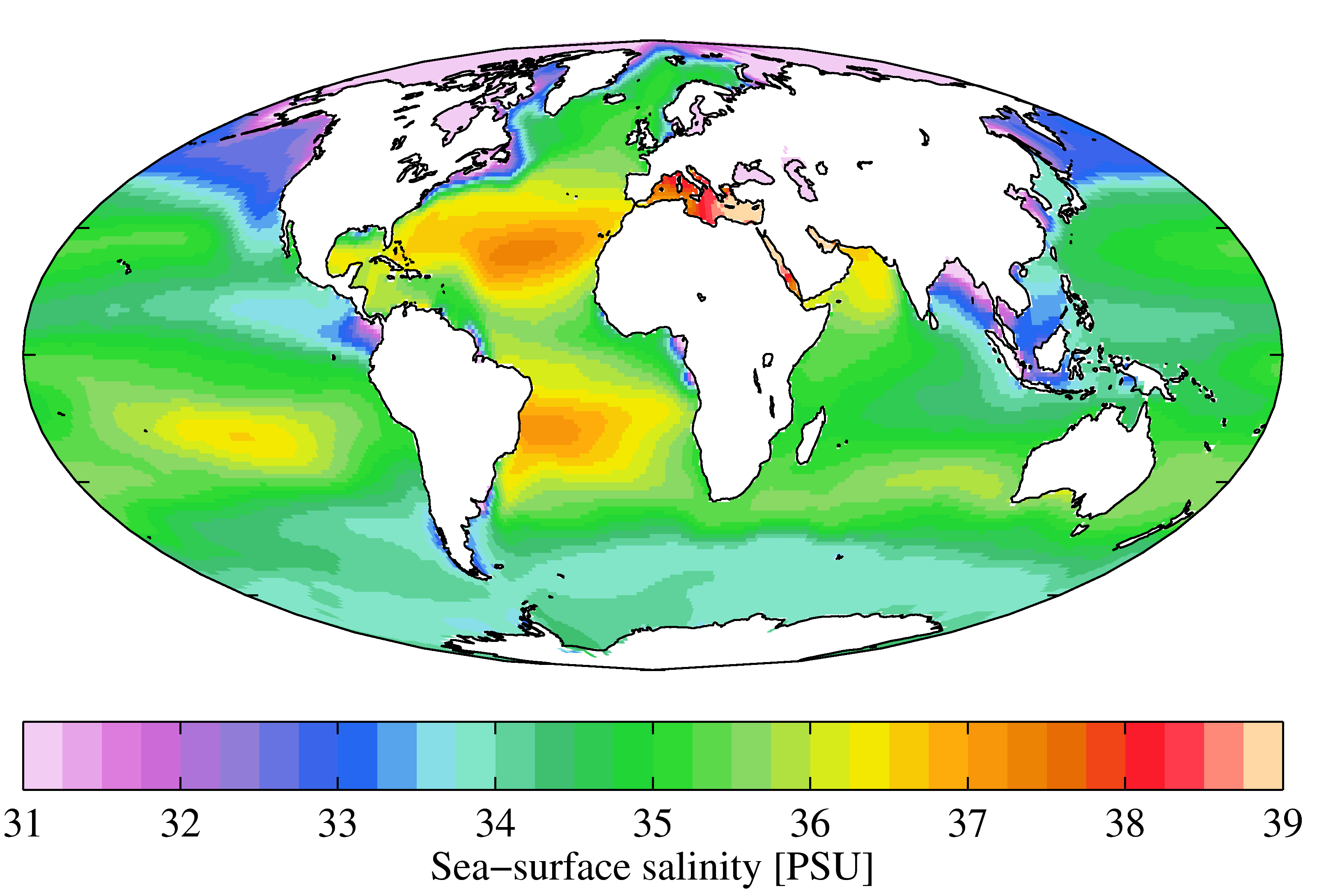 Although the vast majority of seawater has a salinity of between 31 and 38 g/kg, that is 3.1–3.8%, seawater is not uniformly saline throughout the world. Where mixing occurs with freshwater runoff from river mouths, near melting glaciers or vast amounts of precipitation (e.g. Monsoon), seawater can be substantially less saline. The most saline open sea is the
Although the vast majority of seawater has a salinity of between 31 and 38 g/kg, that is 3.1–3.8%, seawater is not uniformly saline throughout the world. Where mixing occurs with freshwater runoff from river mouths, near melting glaciers or vast amounts of precipitation (e.g. Monsoon), seawater can be substantially less saline. The most saline open sea is the
Technical Papers in Marine Science 44, Algorithms for computation of fundamental properties of seawater, ioc-unesco.org, UNESCO 1983
Tables
Tables and software for thermophysical properties of seawater
MIT * {{Authority control Aquatic ecology Chemical oceanography Liquid water Physical oceanography Oceanographical terminology


 Seawater, or salt water, is
Seawater, or salt water, is water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
from a sea or ocean
The ocean (also the sea or the world ocean) is the body of salt water that covers approximately 70.8% of the surface of Earth and contains 97% of Earth's water. An ocean can also refer to any of the large bodies of water into which the wo ...
. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately of dissolved salts (predominantly sodium () and chloride () ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
). The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water and pure water (density 1.0 kg/L at ) because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes
Freezing is a phase transition where a liquid turns into a solid when its temperature is lowered below its freezing point. In accordance with the internationally established definition, freezing means the solidification phase change of a liquid o ...
at about . The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was . Seawater pH is typically limited to a range between 7.5 and 8.4. However, there is no universally accepted reference pH-scale for seawater and the difference between measurements based on different reference scales may be up to 0.14 units.
Properties
Salinity
 Although the vast majority of seawater has a salinity of between 31 and 38 g/kg, that is 3.1–3.8%, seawater is not uniformly saline throughout the world. Where mixing occurs with freshwater runoff from river mouths, near melting glaciers or vast amounts of precipitation (e.g. Monsoon), seawater can be substantially less saline. The most saline open sea is the
Although the vast majority of seawater has a salinity of between 31 and 38 g/kg, that is 3.1–3.8%, seawater is not uniformly saline throughout the world. Where mixing occurs with freshwater runoff from river mouths, near melting glaciers or vast amounts of precipitation (e.g. Monsoon), seawater can be substantially less saline. The most saline open sea is the Red Sea
The Red Sea ( ar, البحر الأحمر - بحر القلزم, translit=Modern: al-Baḥr al-ʾAḥmar, Medieval: Baḥr al-Qulzum; or ; Coptic: ⲫⲓⲟⲙ ⲛ̀ϩⲁϩ ''Phiom Enhah'' or ⲫⲓⲟⲙ ⲛ̀ϣⲁⲣⲓ ''Phiom ǹšari''; ...
, where high rates of evaporation, low precipitation and low river run-off, and confined circulation result in unusually salty water. The salinity in isolated bodies of water can be considerably greater still about ten times higher in the case of the Dead Sea. Historically, several salinity scales were used to approximate the absolute salinity of seawater. A popular scale was the "Practical Salinity Scale" where salinity was measured in "practical salinity units (PSU)". The current standard for salinity is the "Reference Salinity" scale with the salinity expressed in units of "g/kg".
Density
Thedensity
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
of surface seawater ranges from about 1020 to 1029 kg/m3, depending on the temperature and salinity. At a temperature of 25 °C, the salinity of 35 g/kg and 1 atm pressure, the density of seawater is 1023.6 kg/m3. Deep in the ocean, under high pressure, seawater can reach a density of 1050 kg/m3 or higher. The density of seawater also changes with salinity. Brines generated by seawater desalination plants can have salinities up to 120 g/kg. The density of typical seawater brine of 120 g/kg salinity at 25 °C and atmospheric pressure is 1088 kg/m3. Seawater pH is limited to the range 7.5 to 8.4. The speed of sound in seawater is about 1,500 m/s (whereas the speed of sound is usually around 330 m/s in air at roughly 101.3 kPa pressure, 1 atmosphere), and varies with water temperature, salinity, and pressure. The thermal conductivity of seawater is 0.6 W/mK at 25 °C and a salinity of 35 g/kg.
The thermal conductivity decreases with increasing salinity and increases with increasing temperature.
Chemical composition
Seawater contains more dissolved ions than all types of freshwater. However, the ratios of solutes differ dramatically. For instance, although seawater contains about 2.8 times more bicarbonate than river water, the percentage of bicarbonate in seawater as a ratio of ''all'' dissolved ions is far lower than in river water. Bicarbonate ions constitute 48% of river water solutes but only 0.14% for seawater. Differences like these are due to the varying residence times of seawater solutes; sodium and chloride have very long residence times, while calcium (vital for carbonate formation) tends to precipitate much more quickly. The most abundant dissolved ions in seawater are sodium, chloride, magnesium,sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
and calcium. Its osmolarity
Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L). The osmolarity of a solution is usually expressed as Osm/L ...
is about 1000 mOsm/L.
Small amounts of other substances are found, including amino acids at concentrations of up to 2 micrograms of nitrogen atoms per liter, which are thought to have played a key role in the origin of life
In biology, abiogenesis (from a- 'not' + Greek bios 'life' + genesis 'origin') or the origin of life is the natural process by which life has arisen from non-living matter, such as simple organic compounds. The prevailing scientific hypothes ...
.
Microbial components
Research in 1957 by the Scripps Institution of Oceanography sampled water in both pelagic and neritic locations in the Pacific Ocean. Direct microscopic counts and cultures were used, the direct counts in some cases showing up to 10 000 times that obtained from cultures. These differences were attributed to the occurrence of bacteria in aggregates, selective effects of the culture media, and the presence of inactive cells. A marked reduction in bacterial culture numbers was noted below thethermocline
A thermocline (also known as the thermal layer or the metalimnion in lakes) is a thin but distinct layer in a large body of fluid (e.g. water, as in an ocean or lake; or air, e.g. an atmosphere) in which temperature changes more drastically with ...
, but not by direct microscopic observation. Large numbers of spirilli
''Spirillum'' is a genus of Gram-negative bacteria in the family ''Spirillaceae'' of the ''Nitrosomonadales'' of the ''Betaproteobacteria''.Garrity, George M.; Brenner, Don J.; Krieg, Noel R.; Staley, James T. (eds.) (2005). Bergey's Manual of ...
-like forms were seen by microscope but not under cultivation. The disparity in numbers obtained by the two methods is well known in this and other fields. In the 1990s, improved techniques of detection and identification of microbes by probing just small snippets of DNA, enabled researchers taking part in the Census of Marine Life
The Census of Marine Life was a 10-year, US $650 million scientific initiative, involving a global network of researchers in more than 80 nations, engaged to assess and explain the diversity, distribution, and abundance of life in the oceans. Th ...
to identify thousands of previously unknown microbes usually present only in small numbers. This revealed a far greater diversity than previously suspected, so that a litre of seawater may hold more than 20,000 species. Mitchell Sogin Dr. Mitchell Sogin is a distinguished senior scientist at the Marine Biological Laboratory in Woods Hole, Massachusetts, whose research investigates the evolution and diversity of single-celled organisms.
Career
Dr. Sogin obtained a BS in Chemist ...
from the Marine Biological Laboratory
The Marine Biological Laboratory (MBL) is an international center for research and education in biological and environmental science. Founded in Woods Hole, Massachusetts, in 1888, the MBL is a private, nonprofit institution that was independent ...
feels that "the number of different kinds of bacteria in the oceans could eclipse five to 10 million."
Bacteria are found at all depths in the water column, as well as in the sediments, some being aerobic, others anaerobic. Most are free-swimming, but some exist as symbionts within other organisms – examples of these being bioluminescent bacteria. Cyanobacteria played an important role in the evolution of ocean processes, enabling the development of stromatolites
Stromatolites () or stromatoliths () are layered sedimentary formations (microbialite) that are created mainly by photosynthetic microorganisms such as cyanobacteria, sulfate-reducing bacteria, and Pseudomonadota (formerly proteobacteria). Th ...
and oxygen in the atmosphere.
Some bacteria interact with diatoms
A diatom (New Latin, Neo-Latin ''diatoma''), "a cutting through, a severance", from el, διάτομος, diátomos, "cut in half, divided equally" from el, διατέμνω, diatémno, "to cut in twain". is any member of a large group com ...
, and form a critical link in the cycling of silicon in the ocean. One anaerobic species, ''Thiomargarita namibiensis
''Thiomargarita namibiensis'' is a Gram-negative coccoid bacterium, found in the ocean sediments of the continental shelf of Namibia. It is the second largest bacterium ever discovered, as a rule in diameter, but sometimes attaining . Cells of ...
'', plays an important part in the breakdown of hydrogen sulfide eruptions from diatomaceous sediments off the Namibian coast, and generated by high rates of phytoplankton growth in the Benguela Current upwelling zone, eventually falling to the seafloor.
Bacteria-like Archaea surprised marine microbiologists by their survival and thriving in extreme environments, such as the hydrothermal vents on the ocean floor. Alkalotolerant marine bacteria
Marine prokaryotes are marine bacteria and marine archaea. They are defined by their habitat as prokaryotes that live in marine environments, that is, in the saltwater of seas or oceans or the brackish water of coastal estuaries. All cellular ...
such as ''Pseudomonas
''Pseudomonas'' is a genus of Gram-negative, Gammaproteobacteria, belonging to the family Pseudomonadaceae and containing 191 described species. The members of the genus demonstrate a great deal of metabolic diversity and consequently are able t ...
'' and ''Vibrio
''Vibrio'' is a genus of Gram-negative bacteria, possessing a curved-rod (comma) shape, several species of which can cause foodborne infection, usually associated with eating undercooked seafood. Being highly salt tolerant and unable to survive ...
'' spp. survive in a pH range of 7.3 to 10.6, while some species will grow only at pH 10 to 10.6. Archaea also exist in pelagic waters and may constitute as much as half the ocean's biomass, clearly playing an important part in oceanic processes. In 2000 sediments from the ocean floor revealed a species of Archaea that breaks down methane, an important greenhouse gas and a major contributor to atmospheric warming. Some bacteria break down the rocks of the sea floor, influencing seawater chemistry. Oil spills, and runoff containing human sewage and chemical pollutants have a marked effect on microbial life in the vicinity, as well as harbouring pathogens and toxins affecting all forms of marine life. The protist dinoflagellates may at certain times undergo population explosions called blooms or red tide
A harmful algal bloom (HAB) (or excessive algae growth) is an algal bloom that causes negative impacts to other organisms by production of natural algae-produced toxins, mechanical damage to other organisms, or by other means. HABs are sometimes ...
s, often after human-caused pollution. The process may produce metabolites
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, ...
known as biotoxins, which move along the ocean food chain, tainting higher-order animal consumers.
'' Pandoravirus salinus'', a species of very large virus, with a genome much larger than that of any other virus species, was discovered in 2013. Like the other very large viruses '' Mimivirus'' and '' Megavirus'', ''Pandoravirus'' infects amoebas, but its genome, containing 1.9 to 2.5 megabases of DNA, is twice as large as that of ''Megavirus'', and it differs greatly from the other large viruses in appearance and in genome structure.
In 2013 researchers from Aberdeen University
, mottoeng = The fear of the Lord is the beginning of wisdom
, established =
, type = Public research universityAncient university
, endowment = £58.4 million (2021)
, budget ...
announced that they were starting a hunt for undiscovered chemicals in organisms that have evolved in deep sea trenches, hoping to find "the next generation" of antibiotics, anticipating an "antibiotic apocalypse" with a dearth of new infection-fighting drugs. The EU-funded research will start in the Atacama Trench and then move on to search trenches off New Zealand and Antarctica.
The ocean has a long history of human waste disposal on the assumption that its vast size makes it capable of absorbing and diluting all noxious material.
While this may be true on a small scale, the large amounts of sewage routinely dumped has damaged many coastal ecosystems, and rendered them life-threatening. Pathogenic viruses and bacteria occur in such waters, such as '' Escherichia coli'', '' Vibrio cholerae'' the cause of cholera, hepatitis A, hepatitis E
Hepatitis E is inflammation of the liver caused by infection with the hepatitis E virus (HEV); it is a type of viral hepatitis. Hepatitis E has mainly a fecal-oral transmission route that is similar to hepatitis A, although the viruses are unrel ...
and polio, along with protozoans causing giardiasis and cryptosporidiosis
Cryptosporidiosis, sometimes informally called crypto, is a parasitic disease caused by '' Cryptosporidium'', a genus of protozoan parasites in the phylum Apicomplexa. It affects the distal small intestine and can affect the respiratory tra ...
. These pathogens are routinely present in the ballast water of large vessels, and are widely spread when the ballast is discharged.
Origin and history
The water in the sea was thought to come from the Earth's volcanoes, starting 4 billion years ago, released by degassing from molten rock. More recent work suggests much of the Earth's water may come fromcomet
A comet is an icy, small Solar System body that, when passing close to the Sun, warms and begins to release gases, a process that is called outgassing. This produces a visible atmosphere or coma, and sometimes also a tail. These phenomena ...
s.
Scientific theories
A scientific theory is an explanation of an aspect of the natural world and universe that has been repeatedly tested and corroborated in accordance with the scientific method, using accepted protocols of observation, measurement, and evaluatio ...
behind the origins of sea salt started with Sir Edmond Halley
Edmond (or Edmund) Halley (; – ) was an English astronomer, mathematician and physicist. He was the second Astronomer Royal in Britain, succeeding John Flamsteed in 1720.
From an observatory he constructed on Saint Helena in 1676–77, H ...
in 1715, who proposed that salt and other minerals were carried into the sea by rivers after rainfall washed it out of the ground. Upon reaching the ocean, these salts concentrated as more salt arrived over time (see Hydrologic cycle
The water cycle, also known as the hydrologic cycle or the hydrological cycle, is a biogeochemical cycle that describes the continuous movement of water on, above and below the surface of the Earth. The mass of water on Earth remains fairly const ...
). Halley noted that most lakes that don't have ocean outlets (such as the Dead Sea and the Caspian Sea, see endorheic basin
An endorheic basin (; also spelled endoreic basin or endorreic basin) is a drainage basin that normally retains water and allows no outflow to other external bodies of water, such as rivers or oceans, but drainage converges instead into lakes ...
), have high salt content. Halley termed this process "continental weathering".
Halley's theory was partly correct. In addition, sodium leached out of the ocean floor when the ocean formed. The presence of salt's other dominant ion, chloride, results from outgassing
Outgassing (sometimes called offgassing, particularly when in reference to indoor air quality) is the release of a gas that was dissolved, trapped, frozen, or absorbed in some material. Outgassing can include sublimation and evaporation (which ...
of chloride (as hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
) with other gases from Earth's interior via volcanos and hydrothermal vents. The sodium and chloride ions subsequently became the most abundant constituents of sea salt.
Ocean salinity has been stable for billions of years, most likely as a consequence of a chemical/ tectonic system which removes as much salt as is deposited; for instance, sodium and chloride sinks include evaporite
An evaporite () is a water- soluble sedimentary mineral deposit that results from concentration and crystallization by evaporation from an aqueous solution. There are two types of evaporite deposits: marine, which can also be described as ocean ...
deposits, pore-water burial, and reactions with seafloor basalts.
Human impacts
Climate change
In common usage, climate change describes global warming—the ongoing increase in global average temperature—and its effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to ...
, rising levels of carbon dioxide in Earth's atmosphere, excess nutrients, and pollution in many forms are altering global oceanic geochemistry. Rates of change for some aspects greatly exceed those in the historical and recent geological record. Major trends include an increasing acidity
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a ...
, reduced subsurface oxygen in both near-shore and pelagic waters, rising coastal nitrogen levels, and widespread increases in mercury and persistent organic pollutants. Most of these perturbations are tied either directly or indirectly to human fossil fuel combustion, fertilizer, and industrial activity. Concentrations are projected to grow in coming decades, with negative impacts on ocean biota and other marine resources.
One of the most striking features of this is ocean acidification
Ocean acidification is the reduction in the pH value of the Earth’s ocean. Between 1751 and 2021, the average pH value of the ocean surface has decreased from approximately 8.25 to 8.14. The root cause of ocean acidification is carbon dioxid ...
, resulting from increased CO2 uptake of the oceans related to higher atmospheric concentration of CO2 and higher temperatures, because it severely affects coral reefs, mollusks, echinoderms and crustacean
Crustaceans (Crustacea, ) form a large, diverse arthropod taxon which includes such animals as decapods, seed shrimp, branchiopods, fish lice, krill, remipedes, isopods, barnacles, copepods, amphipods and mantis shrimp. The crustacean group can ...
s (see coral bleaching).
Human consumption
Accidentally consuming small quantities of clean seawater is not harmful, especially if the seawater is taken along with a larger quantity of fresh water. However, drinking seawater to maintain hydration is counterproductive; more water must be excreted to eliminate the salt (via urine) than the amount of water obtained from the seawater itself. In normal circumstances, it would be considered ill-advised to consume large amounts of unfiltered seawater. The renal system actively regulates the levels of sodium and chloride in the blood within a very narrow range around 9 g/L (0.9% by mass). In most open waters concentrations vary somewhat around typical values of about 3.5%, far higher than the body can tolerate and most beyond what the kidney can process. A point frequently overlooked in claims that the kidney can excrete NaCl in Baltic concentrations of 2% (in arguments to the contrary) is that the gut cannot absorb water at such concentrations, so that there is no benefit in drinking such water. The salinity of Baltic surface water, however, is never 2%. It is 0,9% or less, and thus never higher than that of bodily fluids. Drinking seawater temporarily increases blood's NaCl concentration. This signals the kidney to excrete sodium, but seawater's sodium concentration is above the kidney's maximum concentrating ability. Eventually the blood's sodium concentration rises to toxic levels, removing water from cells and interfering with nerve conduction, ultimately producing fatal seizure and cardiac arrhythmia. Survival manuals consistently advise against drinking seawater. A summary of 163life raft
A lifeboat or liferaft is a small, rigid or inflatable boat carried for emergency evacuation in the event of a disaster aboard a ship. Lifeboat drills are required by law on larger commercial ships. Rafts ( liferafts) are also used. In the m ...
voyages estimated the risk of death at 39% for those who drank seawater, compared to 3% for those who did not. The effect of seawater intake on rats confirmed the negative effects of drinking seawater when dehydrated.
The temptation to drink seawater was greatest for sailors who had expended their supply of fresh water and were unable to capture enough rainwater for drinking. This frustration was described famously by a line from Samuel Taylor Coleridge's '' The Rime of the Ancient Mariner'':
Although humans cannot survive on seawater, some people claim that up to two cups a day, mixed with fresh water in a 2:3 ratio, produces no ill effect. The French physician Alain Bombard survived an ocean crossing in a small Zodiak rubber boat using mainly raw fish meat, which contains about 40% water (like most living tissues), as well as small amounts of seawater and other provisions harvested from the ocean. His findings were challenged, but an alternative explanation was not given. In his 1948 book '' The Kon-Tiki Expedition'', Thor Heyerdahl reported drinking seawater mixed with fresh in a 2:3 ratio during the 1947 expedition. A few years later, another adventurer, William Willis, claimed to have drunk two cups of seawater and one cup of fresh per day for 70 days without ill effect when he lost part of his water supply.
During the 18th century, Richard Russell advocated the medical use of this practice in the UK, and René Quinton expanded the advocation of this practice to other countries, notably France, in the 20th century. Currently, it is widely practiced in Nicaragua and other countries, supposedly taking advantage of the latest medical discoveries.
Most oceangoing vessels desalinate potable
Drinking water is water that is used in drink or food preparation; potable water is water that is safe to be used as drinking water. The amount of drinking water required to maintain good health varies, and depends on physical activity level, ag ...
water from seawater using processes such as vacuum distillation or multi-stage flash distillation
Multi-stage flash distillation (MSF) is a water desalination process that distills sea water by flashing a portion of the water into steam in multiple stages of what are essentially countercurrent heat exchangers. Current MSF facilities may ha ...
in an evaporator
An evaporator is a device used to turn the liquid form of a chemical substance, such as water, into a vapor.
Uses
Air conditioning and refrigeration
Some air conditioners and refrigerators use a compressed liquid with a low boiling point, su ...
, or, more recently, reverse osmosis. These energy-intensive processes were not usually available during the Age of Sail. Larger sailing warships with large crews, such as Nelson
Nelson may refer to:
Arts and entertainment
* ''Nelson'' (1918 film), a historical film directed by Maurice Elvey
* ''Nelson'' (1926 film), a historical film directed by Walter Summers
* ''Nelson'' (opera), an opera by Lennox Berkeley to a lib ...
's , were fitted with distilling apparatus in their galleys
A galley is a type of ship that is propelled mainly by oars. The galley is characterized by its long, slender hull, shallow draft, and low freeboard (clearance between sea and gunwale). Virtually all types of galleys had sails that could be use ...
.
Animals such as fish, whales, sea turtles, and seabirds, such as penguins and albatrosses, have adapted to living in a high-saline habitat. For example, sea turtles and saltwater crocodiles remove excess salt from their bodies through their tear ducts
The nasolacrimal duct (also called the tear duct) carries tears from the lacrimal sac of the eye into the nasal cavity. The duct begins in the eye socket between the maxillary and lacrimal bones, from where it passes downwards and backwards. ...
.
Mineral extraction
Minerals have been extracted from seawater since ancient times. Currently the four most concentrated metals – Na, Mg, Ca and K – are commercially extracted from seawater. During 2015 in the US 63% of magnesium production came from seawater and brines. Bromine is also produced from seawater in China and Japan. Lithium extraction from seawater was tried in the 1970s, but the tests were soon abandoned. The idea of extracting uranium from seawater has been considered at least from the 1960s, but only a few grams of uranium were extracted in Japan in the late 1990s. The main issue is not one of technological feasibility but that current prices on theuranium market
The uranium market, like all commodity markets, has a history of volatility, moving with the standard forces of supply and demand as well as geopolitical pressures. It has also evolved particularities of its own in response to the unique nature and ...
for uranium from other sources are about three to five times lower than the lowest price achieved by seawater extraction. Similar issues hamper the use of reprocessed uranium
Reprocessed uranium (RepU) is the uranium recovered from nuclear reprocessing, as done commercially in France, the UK and Japan and by nuclear weapons states' military plutonium production programs. This uranium makes up the bulk of the material s ...
and are often brought forth against nuclear reprocessing and the manufacturing of MOX fuel
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material, usually consisting of plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an al ...
as economically unviable.
Standard
ASTM International
ASTM International, formerly known as American Society for Testing and Materials, is an international standards organization that develops and publishes voluntary consensus technical standards for a wide range of materials, products, systems, ...
has an international standard for artificial seawater
Artificial seawater (abbreviated ASW) is a mixture of dissolved mineral salts (and sometimes vitamins) that simulates seawater. Artificial seawater is primarily used in marine biology and in marine and reef aquaria, and allows the easy preparation ...
: ASTM D1141-98 (Original Standard ASTM D1141-52). It is used in many research testing labs as a reproducible solution for seawater such as tests on corrosion, oil contamination, and detergency evaluation.
See also
* * * * * * * * * * * * global ocean salinityReferences
External links
Technical Papers in Marine Science 44, Algorithms for computation of fundamental properties of seawater, ioc-unesco.org, UNESCO 1983
Tables
Tables and software for thermophysical properties of seawater
MIT * {{Authority control Aquatic ecology Chemical oceanography Liquid water Physical oceanography Oceanographical terminology