Quaternary Structure on:
[Wikipedia]
[Google]
[Amazon]
Protein quaternary structure is the fourth (and highest) classification level of
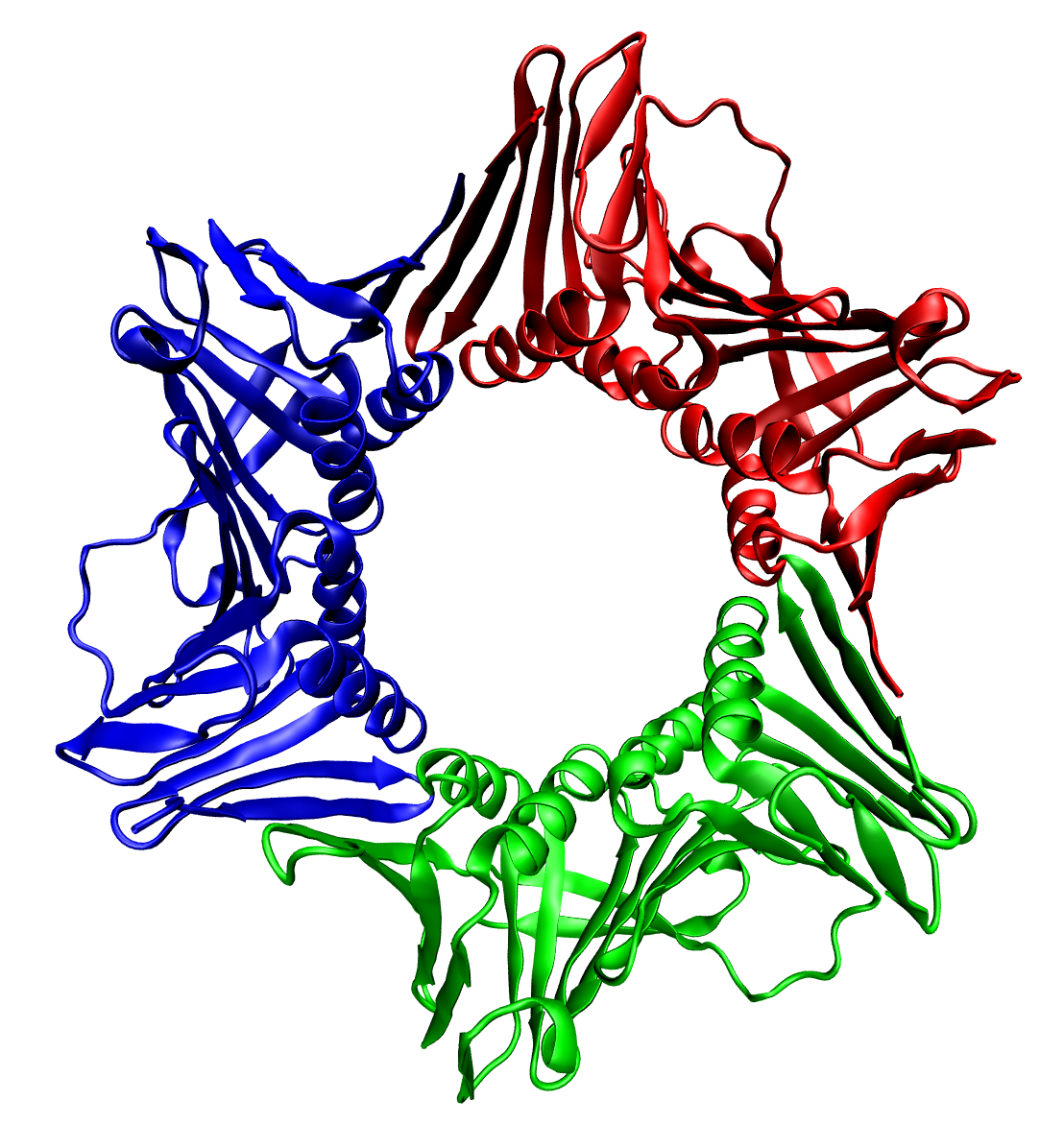 The number of subunits in an oligomeric complex is described using names that end in -mer (Greek for "part, subunit"). Formal and Greco-Latinate names are generally used for the first ten types and can be used for up to twenty subunits, whereas higher order complexes are usually described by the number of subunits, followed by -meric.
:
The number of subunits in an oligomeric complex is described using names that end in -mer (Greek for "part, subunit"). Formal and Greco-Latinate names are generally used for the first ten types and can be used for up to twenty subunits, whereas higher order complexes are usually described by the number of subunits, followed by -meric.
:* ''No known examples''
Although complexes higher than octamers are rarely observed for most proteins, there are some important exceptions. Viral capsids are often composed of multiples of 60 proteins. Several
The Macromolecular Structure Database
(MSD) at the European Bioinformatics Institute (EBI) – Serves a list of the Probable Quaternary Structure (PQS) for every protein in the Protein Data Bank (PDB).
PQS server
– PQS has not been updated since August 2009
– The Protein Interfaces, Surfaces and Assemblies server at the MSD.
EPPIC
– Evolutionary Protein–Protein Interface Classification: evolutionary assessment of interfaces in crystal structures
3D complex
– Structural classification of protein complexes * Proteopedia �
Proteopedia Home Page
The collaborative, 3D encyclopedia of proteins and other molecules. * PDBWiki �
PDBWiki Home Page
– a website for community annotation of PDB structures. *
ProtCID
��a database of similar protein–protein interfaces in crystal structures of homologous proteins. {{Biomolecular structure Protein structure 4 Stereochemistry
protein structure
Protein structure is the three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single amino acid monomer ma ...
. Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains (also referred to as subunits). Protein quaternary structure describes the number and arrangement of multiple folded protein subunit
In structural biology, a protein subunit is a polypeptide chain or single protein molecule that assembles (or "''coassembles''") with others to form a protein complex.
Large assemblies of proteins such as viruses often use a small number of t ...
s in a multi-subunit complex. It includes organizations from simple dimers to large homooligomer
In chemistry and biochemistry, an oligomer () is a molecule that consists of a few repeating units which could be derived, actually or conceptually, from smaller molecules, monomers.Quote: ''Oligomer molecule: A molecule of intermediate relative ...
s and complexes with defined or variable numbers of subunits. In contrast to the first three levels of protein structure, not all proteins will have a quaternary structure since some proteins function as single units. Protein quaternary structure can also refer to biomolecular complex
A biomolecule or biological molecule is a loosely used term for molecules present in organisms that are essential to one or more typically biological processes, such as cell division, morphogenesis, or development. Biomolecules include large ...
es of proteins with nucleic acids and other cofactors
Cofactor may also refer to:
* Cofactor (biochemistry), a substance that needs to be present in addition to an enzyme for a certain reaction to be catalysed
* A domain parameter in elliptic curve cryptography, defined as the ratio between the order ...
.
Description and examples
Many proteins are actually assemblies of multiple polypeptide chains. The quaternary structure refers to the number and arrangement of theprotein subunit
In structural biology, a protein subunit is a polypeptide chain or single protein molecule that assembles (or "''coassembles''") with others to form a protein complex.
Large assemblies of proteins such as viruses often use a small number of t ...
s with respect to one another. Examples of proteins with quaternary structure include hemoglobin, DNA polymerase
A DNA polymerase is a member of a family of enzymes that catalyze the synthesis of DNA molecules from nucleoside triphosphates, the molecular precursors of DNA. These enzymes are essential for DNA replication and usually work in groups to create ...
, ribosomes, antibodies
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the ...
, and ion channel
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ...
s.
Enzymes composed of subunits with diverse functions are sometimes called holoenzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. ...
s, in which some parts may be known as regulatory subunits and the functional core is known as the catalytic subunit. Other assemblies referred to instead as multiprotein complexes also possess quaternary structure. Examples include nucleosomes and microtubules. Changes in quaternary structure can occur through conformational changes within individual subunits or through reorientation of the subunits relative to each other. It is through such changes, which underlie cooperativity
Cooperativity is a phenomenon displayed by systems involving identical or near-identical elements, which act dependently of each other, relative to a hypothetical standard non-interacting system in which the individual elements are acting indepen ...
and allostery in "multimeric" enzymes, that many proteins undergo regulation and perform their physiological function.
The above definition follows a classical approach to biochemistry, established at times when the distinction between a protein and a functional, proteinaceous unit was difficult to elucidate. More recently, people refer to protein–protein interaction when discussing quaternary structure of proteins and consider all assemblies of proteins as protein complex
A protein complex or multiprotein complex is a group of two or more associated polypeptide chains. Protein complexes are distinct from multienzyme complexes, in which multiple catalytic domains are found in a single polypeptide chain.
Protein ...
es.
Nomenclature
 The number of subunits in an oligomeric complex is described using names that end in -mer (Greek for "part, subunit"). Formal and Greco-Latinate names are generally used for the first ten types and can be used for up to twenty subunits, whereas higher order complexes are usually described by the number of subunits, followed by -meric.
:
The number of subunits in an oligomeric complex is described using names that end in -mer (Greek for "part, subunit"). Formal and Greco-Latinate names are generally used for the first ten types and can be used for up to twenty subunits, whereas higher order complexes are usually described by the number of subunits, followed by -meric.
:molecular machine
A molecular machine, nanite, or nanomachine is a molecular component that produces quasi-mechanical movements (output) in response to specific stimuli (input). In cellular biology, macromolecular machines frequently perform tasks essential for l ...
s are also found in the cell, such as the proteasome
Proteasomes are protein complexes which degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks peptide bonds. Enzymes that help such reactions are called proteases.
Proteasomes are part of a major mechanism by wh ...
(four heptameric rings = 28 subunits), the transcription complex and the spliceosome. The ribosome is probably the largest molecular machine, and is composed of many RNA and protein molecules.
In some cases, proteins form complexes that then assemble into even larger complexes. In such cases, one uses the nomenclature, e.g., "dimer of dimers" or "trimer of dimers", to suggest that the complex might dissociate into smaller sub-complexes before dissociating into monomers.
Another distinction often made when referring to oligomers is whether they are homomeric or heteromeric, referring to whether the smaller protein subunits that come together to make the protein complex are the same (homomeric) or different (heteromeric) from each other. For example, two identical protein monomers would come together to form a homo-dimer, whereas two different protein monomers would create a hetero-dimer.
Structure Determination
Protein quaternary structure can be determined using a variety of experimental techniques that require a sample of protein in a variety of experimental conditions. The experiments often provide an estimate of the mass of the native protein and, together with knowledge of the masses and/or stoichiometry of the subunits, allow the quaternary structure to be predicted with a given accuracy. It is not always possible to obtain a precise determination of the subunit composition for a variety of reasons. The number of subunits in a protein complex can often be determined by measuring the hydrodynamic molecular volume or mass of the intact complex, which requires native solution conditions. For ''folded'' proteins, the mass can be inferred from its volume using the partial specific volume of 0.73 ml/g. However, volume measurements are less certain than mass measurements, since ''unfolded'' proteins appear to have a much larger volume than folded proteins; additional experiments are required to determine whether a protein is unfolded or has formed an oligomer.Common techniques used to study protein quaternary structure
* Ultracentrifugation * Surface-induced dissociation mass spectrometry * Coimmunoprecipation *FRET
A fret is any of the thin strips of material, usually metal wire, inserted laterally at specific positions along the neck or fretboard of a stringed instrument. Frets usually extend across the full width of the neck. On some historical instrum ...
* Nuclear Magnetic Resonance (NMR)
Direct mass measurement of intact complexes
* Sedimentation-equilibrium analytical ultracentrifugation * Electrospray mass spectrometry * Mass Spectrometric Immunoassay MSIADirect size measurement of intact complexes
*Static light scattering
Static light scattering is a technique in physical chemistry that measures the intensity of the scattered light to obtain the average molecular weight ''Mw'' of a macromolecule like a polymer or a protein in solution. Measurement of the scattering ...
* Size exclusion chromatography
Size-exclusion chromatography (SEC), also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecule ...
(requires calibration)
* Dual polarisation interferometry
Indirect size measurement of intact complexes
* Sedimentation-velocity analytical ultracentrifugation (measures the translationaldiffusion constant
Fick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equ ...
)
* Dynamic light scattering
Dynamic light scattering (DLS) is a technique in physics that can be used to determine the size distribution profile of small particles in suspension or polymers in solution. In the scope of DLS, temporal fluctuations are usually analyzed using ...
(measures the translational diffusion constant
Fick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equ ...
)
* Pulsed-gradient protein nuclear magnetic resonance (measures the translational diffusion constant
Fick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equ ...
)
* Fluorescence polarization
Fluorescence anisotropy or fluorescence polarization is the phenomenon where the light emitted by a fluorophore has unequal intensities along different axes of polarization. Early pioneers in the field include Aleksander Jablonski, Gregorio Weber ...
(measures the rotational diffusion constant
Fick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equ ...
)
* Dielectric relaxation (measures the rotational diffusion constant
Fick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, . Fick's first law can be used to derive his second law which in turn is identical to the diffusion equ ...
)
* Dual polarisation interferometry (measures the size and the density of the complex)
Methods that measure the mass or volume under unfolding conditions (such as
MALDI-TOF
In mass spectrometry, matrix-assisted laser desorption/ionization (MALDI) is an ionization technique that uses a laser energy absorbing matrix to create ions from large molecules with minimal fragmentation. It has been applied to the analysis of b ...
mass spectrometry and SDS-PAGE) are generally not useful, since non-native conditions usually cause the complex to dissociate into monomers. However, these may sometimes be applicable; for example, the experimenter may apply SDS-PAGE after first treating the intact complex with chemical cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
reagents.
Structure Prediction
Some bioinformatics methods have been developed for predicting the quaternary structural attributes of proteins based on their sequence information by using various modes of pseudo amino acid composition. Protein folding prediction programs used to predict protein tertiary structure have also been expanding to better predict protein quaternary structure. One such development is AlphaFold-Multimer built upon theAlphaFold
AlphaFold is an artificial intelligence (AI) program developed by DeepMind, a subsidiary of Alphabet Inc., Alphabet, which performs Protein structure prediction, predictions of protein structure. The program is designed as a deep learning system. ...
model for predicting protein tertiary structure.
Role in Cell Signaling
Protein quaternary structure also plays an important role in certain cell signaling pathways. The G-protein coupled receptor pathway involves a heterotrimeric protein known as a G-protein. G-proteins contain three distinct subunits known as the G-alpha, G-beta, and G-gamma subunits. When the G-protein is activated, it binds to the G-protein coupled receptor protein and the cell signaling pathway is initiated. Another example is the receptor tyrosine kinase (RTK) pathway, which is initiated by the dimerization of two receptor tyrosine kinase monomers. When the dimer is formed, the two kinases can phosphorylate each other and initiate a cell signaling pathway.Protein–protein interactions
Proteins are capable of forming very tight complexes. For example, ribonuclease inhibitor binds toribonuclease A
Pancreatic ribonuclease family (, ''RNase'', ''RNase I'', ''RNase A'', ''pancreatic RNase'', ''ribonuclease I'', ''endoribonuclease I'', ''ribonucleic phosphatase'', ''alkaline ribonuclease'', ''ribonuclease'', ''gene S glycoproteins'', ''Ceratit ...
with a roughly 20 fM dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex f ...
. Other proteins have evolved to bind specifically to unusual moieties on another protein, e.g., biotin groups (avidin), phosphorylated tyrosines (SH2 domains) or proline-rich segments (SH3 domains). Protein-protein interactions can be engineered to favor certain oligomerization states.
Intragenic complementation
When multiple copies of a polypeptide encoded by a gene form a quaternary complex, this protein structure is referred to as a multimer. When a multimer is formed from polypeptides produced by two different mutantallele
An allele (, ; ; modern formation from Greek ἄλλος ''állos'', "other") is a variation of the same sequence of nucleotides at the same place on a long DNA molecule, as described in leading textbooks on genetics and evolution.
::"The chro ...
s of a particular gene, the mixed multimer may exhibit greater functional activity than the unmixed multimers formed by each of the mutants alone. In such a case, the phenomenon is referred to as intragenic complementation
Epistasis is a phenomenon in genetics in which the effect of a gene mutation is dependent on the presence or absence of mutations in one or more other genes, respectively termed modifier genes. In other words, the effect of the mutation is dep ...
(also called inter-allelic complementation). Intragenic complementation appears to be common and has been studied in many different genes in a variety of organisms including the fungi ''Neurospora crassa
''Neurospora crassa'' is a type of red bread mold of the phylum Ascomycota. The genus name, meaning "nerve spore" in Greek, refers to the characteristic striations on the spores. The first published account of this fungus was from an infestation ...
'', ''Saccharomyces cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been ...
'' and ''Schizosaccharomyces pombe
''Schizosaccharomyces pombe'', also called "fission yeast", is a species of yeast used in traditional brewing and as a model organism in molecular and cell biology. It is a unicellular eukaryote, whose cells are rod-shaped. Cells typically measur ...
''; the bacterium '' Salmonella typhimurium''; the virus bacteriophage T4
Escherichia virus T4 is a species of bacteriophages that infect ''Escherichia coli'' bacteria. It is a double-stranded DNA virus in the subfamily '' Tevenvirinae'' from the family Myoviridae. T4 is capable of undergoing only a lytic lifecycl ...
, an RNA virus, and humans. The intermolecular forces likely responsible for self-recognition and multimer formation were discussed by Jehle.
Assembly
Direct interaction of two nascent proteins emerging from nearby ribosomes appears to be a general mechanism for oligomer formation. Hundreds of protein oligomers were identified that assemble in human cells by such an interaction. The most prevalent form of interaction was between the N-terminal regions of the interacting proteins. Dimer formation appears to be able to occur independently of dedicated assembly machines.See also
*Structural biology
Structural biology is a field that is many centuries old which, and as defined by the Journal of Structural Biology, deals with structural analysis of living material (formed, composed of, and/or maintained and refined by living cells) at every le ...
* Nucleic acid quaternary structure
Nucleic acid quaternary structure refers to the interactions between separate nucleic acid molecules, or between nucleic acid molecules and proteins. The concept is analogous to protein quaternary structure, but as the analogy is not perfect, the ...
* Multiprotein complex
* Biomolecular complex
A biomolecule or biological molecule is a loosely used term for molecules present in organisms that are essential to one or more typically biological processes, such as cell division, morphogenesis, or development. Biomolecules include large ...
* Oligomers
Notes
References
External links
The Macromolecular Structure Database
(MSD) at the European Bioinformatics Institute (EBI) – Serves a list of the Probable Quaternary Structure (PQS) for every protein in the Protein Data Bank (PDB).
PQS server
– PQS has not been updated since August 2009
– The Protein Interfaces, Surfaces and Assemblies server at the MSD.
EPPIC
– Evolutionary Protein–Protein Interface Classification: evolutionary assessment of interfaces in crystal structures
3D complex
– Structural classification of protein complexes * Proteopedia �
Proteopedia Home Page
The collaborative, 3D encyclopedia of proteins and other molecules. * PDBWiki �
PDBWiki Home Page
– a website for community annotation of PDB structures. *
ProtCID
The Protein Common Interface Database (ProtCID) is a database of similar protein-protein interfaces in crystal structures of homologous proteins.
Its main goal is to identify and cluster homodimeric and heterodimeric interfaces observed in mul ...
�ProtCID
��a database of similar protein–protein interfaces in crystal structures of homologous proteins. {{Biomolecular structure Protein structure 4 Stereochemistry