Posttranslational Modification on:
[Wikipedia]
[Google]
[Amazon]
 Post-translational modification (PTM) is the covalent and generally enzymatic modification of
Post-translational modification (PTM) is the covalent and generally enzymatic modification of
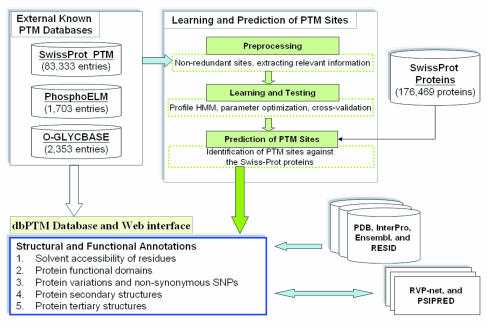 Protein sequences contain sequence motifs that are recognized by modifying enzymes, and which can be documented or predicted in PTM databases. With the large number of different modifications being discovered, there is a need to document this sort of information in databases. PTM information can be collected through experimental means or predicted from high-quality, manually curated data. Numerous databases have been created, often with a focus on certain taxonomic groups (e.g. human proteins) or other features.
Protein sequences contain sequence motifs that are recognized by modifying enzymes, and which can be documented or predicted in PTM databases. With the large number of different modifications being discovered, there is a need to document this sort of information in databases. PTM information can be collected through experimental means or predicted from high-quality, manually curated data. Numerous databases have been created, often with a focus on certain taxonomic groups (e.g. human proteins) or other features.
PhosphoSitePlus
– A database of comprehensive information and tools for the study of mammalian protein post-translational modification * ProteomeScout – A database of proteins and post-translational modifications experimentally *
Uniprot
has PTM information although that may be less comprehensive than in more specialized databases.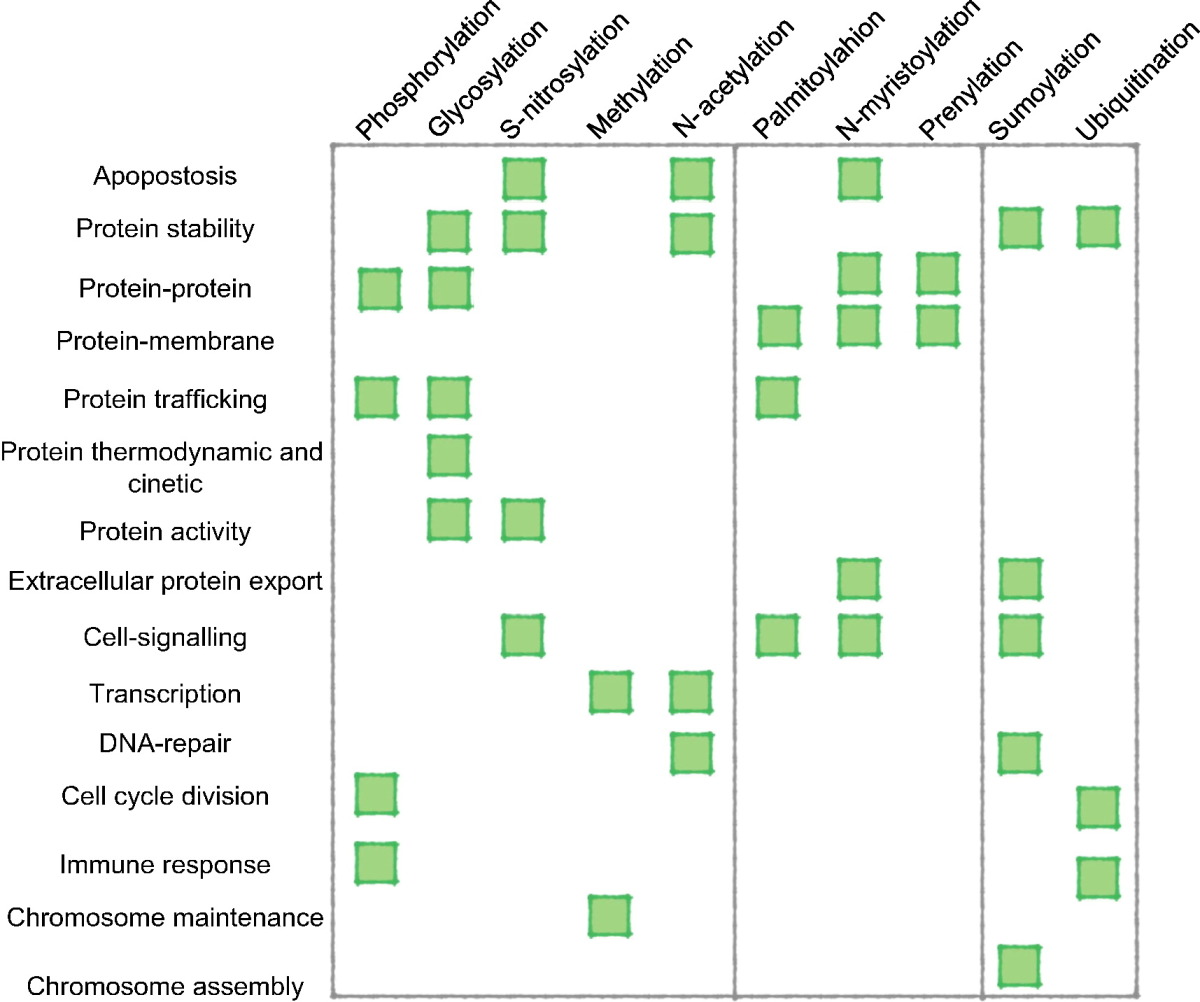
The ''O''-GlcNAc Database
- A curated database for protein O-GlcNAcylation and referencing more than 14 000 protein entries and 10 000 ''O''-GlcNAc sites.
dbPTM - database of protein post-translational modifications
(
List of posttranslational modifications in ExPASy
Browse SCOP domains by PTM
— from the dcGO database
Statistics of each post-translational modification from the Swiss-Prot database
(Wayback Machine copy)
AutoMotif Server
A Computational Protocol for Identification of Post-Translational Modifications in Protein Sequences
Functional analyses for site-specific phosphorylation of a target protein in cells
* ttp://www.cytoskeleton.com/about-signal-seeker-ptm-detection Overview and description of commonly used post-translational modification detection techniques {{DEFAULTSORT:Posttranslational Modification Gene expression Protein structure Protein biosynthesis Cell biology
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles ...
. Proteins are synthesized by ribosomes translating
Translation is the communication of the meaning of a source-language text by means of an equivalent target-language text. The English language draws a terminological distinction (which does not exist in every language) between ''transl ...
mRNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of synthesizing a protein.
mRNA is created during the ...
into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormone
A prohormone is a committed precursor of a hormone consisting of peptide hormones synthesized together that has a minimal hormonal effect by itself because of its expression-suppressing structure, often created by protein folding and binding additi ...
s are converted to hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are require ...
s.
Post-translational modifications can occur on the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a ...
s or at the protein's C- or N- termini. They can extend the chemical repertoire of the 20 standard amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
s by modifying an existing functional group or introducing a new one such as phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
. Phosphorylation is a highly effective mechanism for regulating the activity of enzymes and is the most common post-translational modification. Many eukaryotic
Eukaryotes () are organisms whose Cell (biology), cells have a cell nucleus, nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the ...
and prokaryotic proteins also have carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or m ...
molecules attached to them in a process called glycosylation, which can promote protein folding and improve stability as well as serving regulatory functions. Attachment of lipid
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids includ ...
molecules, known as lipidation, often targets a protein or part of a protein attached to the cell membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment ( ...
.
Other forms of post-translational modification consist of cleaving peptide bonds, as in processing a propeptide to a mature form or removing the initiator methionine residue. The formation of disulfide bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
s from cysteine residues may also be referred to as a post-translational modification. For instance, the peptide hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are require ...
insulin is cut twice after disulfide bonds are formed, and a propeptide is removed from the middle of the chain; the resulting protein consists of two polypeptide chains connected by disulfide bonds.
Some types of post-translational modification are consequences of oxidative stress
Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal ...
. Carbonylation is one example that targets the modified protein for degradation and can result in the formation of protein aggregates. Specific amino acid modifications can be used as biomarker
In biomedical contexts, a biomarker, or biological marker, is a measurable indicator of some biological state or condition. Biomarkers are often measured and evaluated using blood, urine, or soft tissues to examine normal biological processes, p ...
s indicating oxidative damage.
Sites that often undergo post-translational modification are those that have a functional group that can serve as a nucleophile in the reaction: the hydroxyl groups of serine, threonine, and tyrosine; the amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
forms of lysine, arginine, and histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the d ...
; the thiolate
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
anion of cysteine; the carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carboxylat ...
s of aspartate and glutamate; and the N- and C-termini. In addition, although the amide of asparagine is a weak nucleophile, it can serve as an attachment point for glycans. Rarer modifications can occur at oxidized methionines and at some methylene groups in side chains.
Post-translational modification of proteins can be experimentally detected by a variety of techniques, including mass spectrometry, Eastern blotting, and Western blotting
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detect ...
. Additional methods are provided in the #External links section.
PTMs involving addition of functional groups
Addition by an enzyme ''in vivo''
Hydrophobic groups for membrane localization
* myristoylation (a type ofacylation
In chemistry, acylation (or alkanoylation) is the chemical reaction in which an acyl group () is added to a compound. The compound providing the acyl group is called the acylating agent.
Because they form a strong electrophile when treated with ...
), attachment of myristate, a C14 saturated acid
* palmitoylation (a type of acylation), attachment of palmitate
Palmitic acid (hexadecanoic acid in IUPAC nomenclature) is a fatty acid with a 16-carbon chain. It is the most common saturated fatty acid found in animals, plants and microorganisms.Gunstone, F. D., John L. Harwood, and Albert J. Dijkstra. The L ...
, a C16 saturated acid
* isoprenylation
Prenylation (also known as isoprenylation or lipidation) is the addition of hydrophobic molecules to a protein or a biomolecule. It is usually assumed that prenyl groups (3-methylbut-2-en-1-yl) facilitate attachment to cell membranes, similar to ...
or prenylation, the addition of an isoprenoid group (e.g. farnesol and geranylgeraniol)
** farnesylation
Prenylation (also known as isoprenylation or lipidation) is the addition of hydrophobic molecules to a protein or a biomolecule. It is usually assumed that prenyl groups (3-methylbut-2-en-1-yl) facilitate attachment to cell membranes, similar to ...
** geranylgeranylation Geranylgeranylation is a form of prenylation, which is a post-translational modification of proteins that involves the attachment of one or two 20-carbon lipophilic geranylgeranyl isoprene units from geranylgeranyl diphosphate to one or two cysteine ...
* glypiation Glypiation is the addition by covalent bonding of a glycosylphosphatidylinositol (GPI) anchor and is a common post-translational modification that localizes proteins to cell membranes. This special kind of glycosylation is widely detected on surfac ...
, glycosylphosphatidylinositol (GPI) anchor formation via an amide bond to C-terminal tail
Cofactors for enhanced enzymatic activity
* lipoylation (a type of acylation), attachment of a lipoate (C8) functional group * flavin moiety ( FMN or FAD) may be covalently attached * heme C attachment viathioether
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A su ...
bonds with cysteines
* phosphopantetheinylation, the addition of a 4'-phosphopantetheinyl moiety from coenzyme A, as in fatty acid, polyketide, non-ribosomal peptide and leucine biosynthesis
* retinylidene Schiff base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldimine ...
formation
Modifications of translation factors
* diphthamide formation (on a histidine found ineEF2
Eukaryotic elongation factor 2 is a protein that in humans is encoded by the ''EEF2'' gene. It is the archaeal and eukaryotic counterpart of bacterial EF-G.
This gene encodes a member of the GTP-binding translation elongation factor family. Thi ...
)
* ethanolamine phosphoglycerol attachment (on glutamate found in eEF1α)
* hypusine
Hypusine is an uncommon amino acid found in all eukaryotes and in some archaea, but not in bacteria. The only known proteins containing the hypusine residue is eukaryotic translation initiation factor 5A (eIF-5A) and a similar protein found in ...
formation (on conserved lysine of eIF5A
Eukaryotic translation initiation factor 5A-1 is a protein that in humans is encoded by the ''EIF5A'' gene.
It is the only known protein to contain the unusual amino acid hypusine 'N''ε-(4-amino-2-hydroxybutyl)-lysine which is synthesized on e ...
(eukaryotic) and aIF5A (archaeal))
* beta-Lysine addition on a conserved lysine of the elongation factor P (EFP) in most bacteria. EFP is a homolog to eIF5A
Eukaryotic translation initiation factor 5A-1 is a protein that in humans is encoded by the ''EIF5A'' gene.
It is the only known protein to contain the unusual amino acid hypusine 'N''ε-(4-amino-2-hydroxybutyl)-lysine which is synthesized on e ...
(eukaryotic) and aIF5A (archaeal) (see above).
Smaller chemical groups
*acylation
In chemistry, acylation (or alkanoylation) is the chemical reaction in which an acyl group () is added to a compound. The compound providing the acyl group is called the acylating agent.
Because they form a strong electrophile when treated with ...
, e.g. ''O''-acylation (esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
), ''N''-acylation (amides
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is ...
), ''S''-acylation (thioesters
In organic chemistry, thioesters are organosulfur compounds with the functional group . They are analogous to carboxylate esters () with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester, as implied by ...
)
** acetylation, the addition of an acetyl group, either at the N-terminus of the protein or at lysine residues. The reverse is called deacetylation.
** formylation
* alkylation, the addition of an alkyl group, e.g. methyl, ethyl
** methylation the addition of a methyl group, usually at lysine or arginine residues. The reverse is called demethylation Demethylation is the chemical process resulting in the removal of a methyl group (CH3) from a molecule. A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen at ...
.
* amidation at C-terminus. Formed by oxidative dissociation of a C-terminal Gly residue.
* amide bond formation
** amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
addition
*** arginylation
Arginylation is a post-translational modification in which proteins are modified by the addition of arginine (Arg) at the N-terminal amino group or side chains of reactive amino acids by the enzyme, arginyltransferase (ATE1). Recent studies hav ...
, a tRNA
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino ...
-mediation addition
*** polyglutamylation, covalent linkage of glutamic acid residues to the N-terminus of tubulin and some other proteins. (See tubulin polyglutamylase)
*** polyglycylation, covalent linkage of one to more than 40 glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid ( carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinog ...
residues to the tubulin
Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoske ...
C-terminal tail
* butyrylation
* gamma-carboxylation dependent on Vitamin K
* glycosylation, the addition of a glycosyl group to either arginine, asparagine, cysteine, hydroxylysine, serine, threonine, tyrosine, or tryptophan resulting in a glycoprotein. Distinct from glycation, which is regarded as a nonenzymatic attachment of sugars.
** ''O''-GlcNAc, addition of ''N''-acetylglucosamine to serine or threonine residues in a β-glycosidic linkage
** polysialylation, addition of polysialic acid Polysialic acid is an unusual posttranslational modification that occurs on neural cell adhesion molecules (NCAM). Polysialic acid is considerably anionic. This strong negative charge gives this modification the ability to change the protein's surfa ...
, PSA, to NCAM
Neural cell adhesion molecule (NCAM), also called CD56, is a homophilic binding glycoprotein expressed on the surface of neurons, glia and skeletal muscle. Although CD56 is often considered a marker of neural lineage commitment due to its discove ...
* malonylation
* hydroxylation: addition of an oxygen atom to the side-chain of a Pro or Lys residue
* iodination: addition of an iodine atom to the aromatic ring of a tyrosine residue (e.g. in thyroglobulin)
* nucleotide addition such as ADP-ribosylation
* phosphate ester (''O''-linked) or phosphoramidate
Phosphoramidates (sometimes also called amidophosphates) are a class of phosphorus compounds structurally related to phosphates (or organophosphates) via the substitution of an OR for a NR2. They are derivatives of phosphoramidic acids O=P(OH)(NR2 ...
(''N''-linked) formation
** phosphorylation, the addition of a phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
group, usually to serine, threonine, and tyrosine (''O''-linked), or histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the d ...
(''N''-linked)
** adenylylation
Adenylylation, more commonly known as AMPylation, is a process in which an adenosine monophosphate (AMP) molecule is covalently attached to the amino acid side chain of a protein. This covalent addition of AMP to a hydroxyl side chain of the prote ...
, the addition of an adenylyl moiety, usually to tyrosine (''O''-linked), or histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the d ...
and lysine (''N''-linked)
** uridylylation, the addition of an uridylyl-group (i.e. uridine monophosphate, UMP), usually to tyrosine
* propionylation
* pyroglutamate formation
* ''S''-glutathionylation
* ''S''-nitrosylation
* ''S''-sulfenylation (''aka'' ''S''-sulphenylation), reversible covalent addition of one oxygen atom to the thiol group of a cysteine residue
* ''S''-sulfinylation, normally irreversible covalent addition of two oxygen atoms to the thiol group of a cysteine residue
* ''S''-sulfonylation, normally irreversible covalent addition of three oxygen atoms to the thiol group of a cysteine residue, resulting in the formation of a cysteic acid
Cysteic acid also known as 3-sulfo--alanine is the organic compound with the formula HO3SCH2CH(NH2)CO2H. It is often referred to as cysteate, which near neutral pH takes the form −O3SCH2CH(NH3+)CO2−.
It is an amino acid generated by oxidation ...
residue
* succinylation addition of a succinyl group to lysine
* sulfation
Sulfation is the chemical reaction that entails the addition of SO3 group. In principle, many sulfations would involve reactions of sulfur trioxide (SO3). In practice, most sulfations are effected less directly. Regardless of the mechanism, the ...
, the addition of a sulfate group to a tyrosine.
Non-enzymatic additions ''in vivo''
* glycation, the addition of a sugar molecule to a protein without the controlling action of an enzyme. * carbamylation the addition of Isocyanic acid to a protein's N-terminus or the side-chain of Lys. * carbonylation the addition of carbon monoxide to other organic/inorganic compounds. * spontaneous isopeptide bond formation, as found in many surface proteins of Gram-positive bacteria.Non-enzymatic additions ''in vitro''
* biotinylation: covalent attachment of a biotin moiety using a biotinylation reagent, typically for the purpose of labeling a protein. * carbamylation: the addition of Isocyanic acid to a protein's N-terminus or the side-chain of Lys or Cys residues, typically resulting from exposure to urea solutions. * oxidation: addition of one or more Oxygen atoms to a susceptible side-chain, principally of Met, Trp, His or Cys residues. Formation ofdisulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
bonds between Cys residues.
* pegylation: covalent attachment of polyethylene glycol
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular we ...
(PEG) using a pegylation reagent, typically to the N-terminus or the side-chains of Lys residues. Pegylation is used to improve the efficacy of protein pharmaceuticals.
Conjugation with other proteins or peptides
*ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
ation, the covalent linkage to the protein ubiquitin.
* SUMOylation, the covalent linkage to the SUMO protein (Small Ubiquitin-related MOdifier)
* neddylation, the covalent linkage to the Nedd protein
* ISGylation, the covalent linkage to the ISG15 protein (Interferon-Stimulated Gene 15)
* pupylation, the covalent linkage to the prokaryotic ubiquitin-like protein
Chemical modification of amino acids
* citrullination, or deimination, the conversion of arginine to citrulline *deamidation
Deamidation is a chemical reaction in which an amide functional group in the side chain of the amino acids asparagine or glutamine is removed or converted to another functional group. Typically, asparagine is converted to aspartic acid or isoaspa ...
, the conversion of glutamine
Glutamine (symbol Gln or Q) is an α-amino acid that is used in the biosynthesis of proteins. Its side chain is similar to that of glutamic acid, except the carboxylic acid group is replaced by an amide. It is classified as a charge-neutral ...
to glutamic acid or asparagine to aspartic acid
* eliminylation, the conversion to an alkene by beta-elimination of phosphothreonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO� ...
and phosphoserine
Phosphoserine (abbreviated as SEP or J) is an ester of serine and phosphoric acid. Phosphoserine is a component of many proteins as the result of posttranslational modifications. The phosphorylation of the alcohol functional group in serine to pro ...
, or dehydration
In physiology, dehydration is a lack of total body water, with an accompanying disruption of metabolic processes. It occurs when free water loss exceeds free water intake, usually due to exercise, disease, or high environmental temperature. Mil ...
of threonine and serine
Structural changes
*disulfide bridge
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
s, the covalent linkage of two cysteine amino acids
* proteolytic cleavage
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, ...
, cleavage of a protein at a peptide bond
* isoaspartate
Isoaspartic acid (isoaspartate, isoaspartyl, β-aspartate) is an aspartic acid residue isomeric to the typical α peptide linkage. It is a β-amino acid, with the side chain carboxyl moved to the backbone. Such a change is caused by a chemical re ...
formation, via the cyclisation of asparagine or aspartic acid amino-acid residues
* racemization In chemistry, racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic (optically inactive) form. This creates a 1:1 molar ratio of enantiomers and is referred too as a racemic mixture (i.e. conta ...
** of serine by protein-serine epimerase
** of alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side ...
in dermorphin
Dermorphin is a hepta-peptide first isolated from the skin of South American frogs belonging to the genus ''Phyllomedusa''. The peptide is a natural opioid that binds as an agonist with high potency and selectivity to mu Opioid receptors. Dermorp ...
, a frog opioid peptide
** of methionine in deltorphin
Deltorphin, also known as deltorphin A and dermenkephalin, is a natural product, naturally occurring, exogenous opioid peptide, opioid heptapeptide and thus, exorphin, with the amino acid sequence Tyr-D-Met-Phe-His-Leu-Met-Asp-NH2. Along with the ...
, also a frog opioid peptide
* protein splicing
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respond ...
, self-catalytic removal of inteins analogous to mRNA processing
Statistics
Common PTMs by frequency
In 2011, statistics of each post-translational modification experimentally and putatively detected have been compiled using proteome-wide information from the Swiss-Prot database. The 10 most common experimentally found modifications were as follows:Common PTMs by residue
Some common post-translational modifications to specific amino-acid residues are shown below. Modifications occur on the side-chain unless indicated otherwise.Databases and tools
 Protein sequences contain sequence motifs that are recognized by modifying enzymes, and which can be documented or predicted in PTM databases. With the large number of different modifications being discovered, there is a need to document this sort of information in databases. PTM information can be collected through experimental means or predicted from high-quality, manually curated data. Numerous databases have been created, often with a focus on certain taxonomic groups (e.g. human proteins) or other features.
Protein sequences contain sequence motifs that are recognized by modifying enzymes, and which can be documented or predicted in PTM databases. With the large number of different modifications being discovered, there is a need to document this sort of information in databases. PTM information can be collected through experimental means or predicted from high-quality, manually curated data. Numerous databases have been created, often with a focus on certain taxonomic groups (e.g. human proteins) or other features.
List of resources
PhosphoSitePlus
– A database of comprehensive information and tools for the study of mammalian protein post-translational modification * ProteomeScout – A database of proteins and post-translational modifications experimentally *
Human Protein Reference Database
The Human Protein Reference Database (HPRD) is a protein database accessible through the Internet. It is closely associated with the premier Indian Non-Profit research organisation Institute of Bioinformatics (IOB), Bangalore. This database is a c ...
– A database for different modifications and understand different proteins, their class, and function/process related to disease causing proteins
* PROSITE
PROSITE is a protein database. It consists of entries describing the protein families, domains and functional sites as well as amino acid patterns and profiles in them. These are manually curated by a team of the Swiss Institute of Bioinformatic ...
– A database of Consensus patterns for many types of PTM's including sites
* Protein Information Resource (PIR) – A database to acquire a collection of annotations and structures for PTMs.
* dbPTM – A database that shows different PTM's and information regarding their chemical components/structures and a frequency for amino acid modified site
Uniprot
has PTM information although that may be less comprehensive than in more specialized databases.

The ''O''-GlcNAc Database
- A curated database for protein O-GlcNAcylation and referencing more than 14 000 protein entries and 10 000 ''O''-GlcNAc sites.
Tools
List of software for visualization of proteins and their PTMs *PyMOL
PyMOL is an open source but proprietary molecular visualization system created by Warren Lyford DeLano. It was commercialized initially by DeLano Scientific LLC, which was a private software company dedicated to creating useful tools that becom ...
– introduce a set of common PTM's into protein models
* AWESOME – Interactive tool to see the role of single nucleotide polymorphisms to PTM's
* Chimera
Chimera, Chimaera, or Chimaira (Greek for " she-goat") originally referred to:
* Chimera (mythology), a fire-breathing monster of Ancient Lycia said to combine parts from multiple animals
* Mount Chimaera, a fire-spewing region of Lycia or Cilici ...
– Interactive Database to visualize molecules
Case examples
* Cleavage and formation ofdisulfide bridge
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
s during the production of insulin
* PTM of histone
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn a ...
s as regulation of transcription: RNA polymerase control by chromatin structure
* PTM of RNA polymerase II as regulation of transcription
* Cleavage of polypeptide chains as crucial for lectin specificity
See also
* Protein targeting * Post-translational regulationReferences
External links
dbPTM - database of protein post-translational modifications
(
Wayback Machine
The Wayback Machine is a digital archive of the World Wide Web founded by the Internet Archive, a nonprofit based in San Francisco, California. Created in 1996 and launched to the public in 2001, it allows the user to go "back in time" and see ...
copy)
List of posttranslational modifications in ExPASy
Browse SCOP domains by PTM
— from the dcGO database
Statistics of each post-translational modification from the Swiss-Prot database
(Wayback Machine copy)
AutoMotif Server
A Computational Protocol for Identification of Post-Translational Modifications in Protein Sequences
Functional analyses for site-specific phosphorylation of a target protein in cells
* ttp://www.cytoskeleton.com/about-signal-seeker-ptm-detection Overview and description of commonly used post-translational modification detection techniques {{DEFAULTSORT:Posttranslational Modification Gene expression Protein structure Protein biosynthesis Cell biology