polythiophene on:
[Wikipedia]
[Google]
[Amazon]
 Polythiophenes (PTs) are
Polythiophenes (PTs) are

 One undesirable feature of 3-alkylthiophenes is the variable regioregularity of the polymer. Focusing on the polymer
One undesirable feature of 3-alkylthiophenes is the variable regioregularity of the polymer. Focusing on the polymer
 The
The
 In terms of mechanism, the oxidative polymerization using ferric chloride, a radical pathway has been proposed. Niemi ''et al.'' reported that polymerization was only observed in solvents where the catalyst was either partially or completely insoluble (chloroform,
In terms of mechanism, the oxidative polymerization using ferric chloride, a radical pathway has been proposed. Niemi ''et al.'' reported that polymerization was only observed in solvents where the catalyst was either partially or completely insoluble (chloroform,
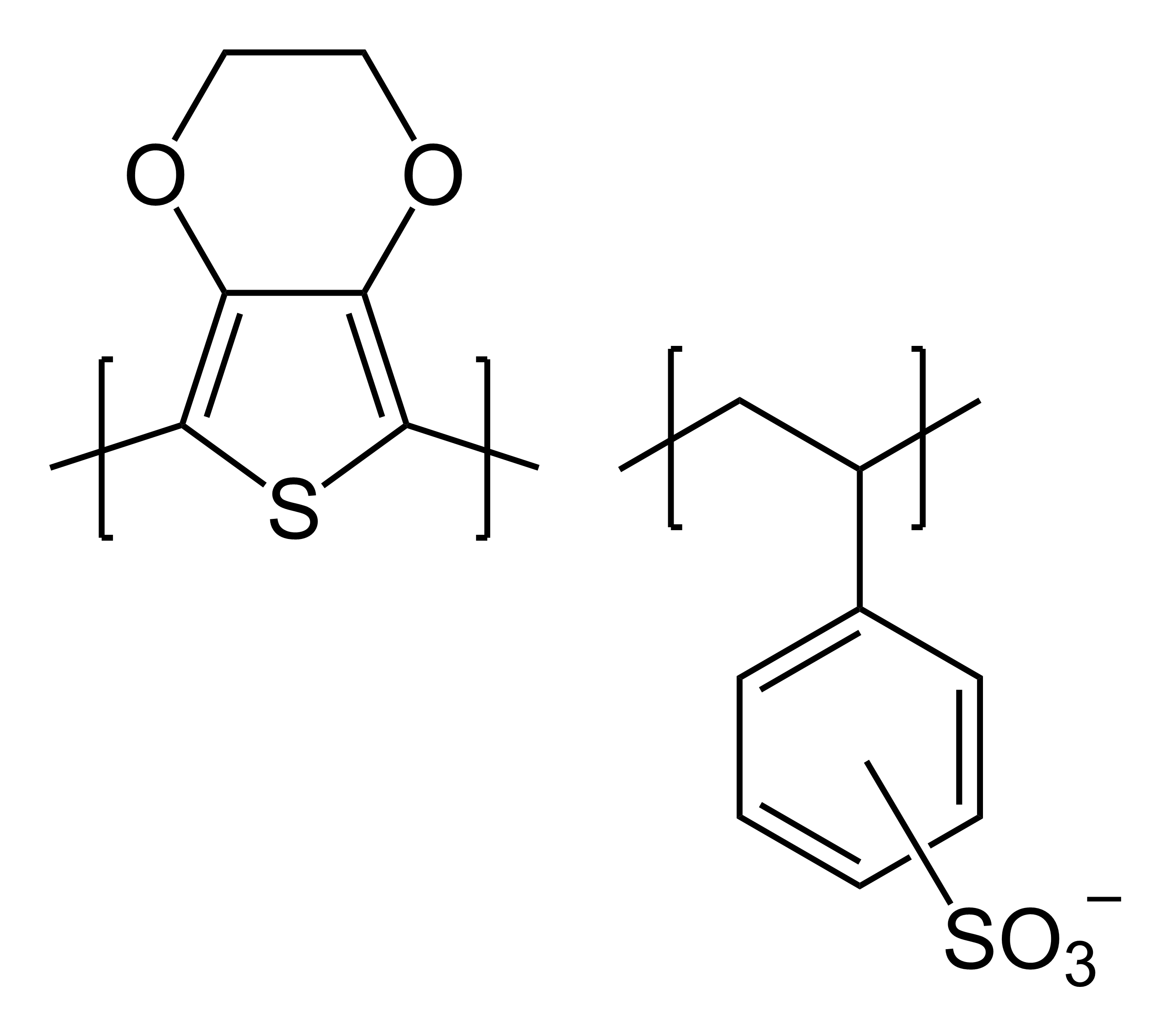 As an example of a static application, poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate) (PEDOT-PSS) product ("Clevios P") from
As an example of a static application, poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate) (PEDOT-PSS) product ("Clevios P") from
''Synthetic Metals''
(journal). ISSN 0379-6779 * * * *
 Polythiophenes (PTs) are
Polythiophenes (PTs) are polymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
ized thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reacti ...
s, a sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.
PTs become conductive
In physics and electrical engineering, a conductor is an object or type of material that allows the flow of charge (electric current) in one or more directions. Materials made of metal are common electrical conductors. Electric current is gene ...
when oxidized. The electrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows ...
results from the delocalization of electrons along the polymer backbone. Conductivity however is not the only interesting property resulting from electron delocalization. The optical properties of these materials respond to environmental stimuli, with dramatic color shifts in response to changes in solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
, temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
, applied potential, and binding to other molecules. Changes in both color and conductivity are induced by the same mechanism, twisting of the polymer backbone and disrupting conjugation, making conjugated polymers attractive as sensors
A sensor is a device that produces an output signal for the purpose of sensing a physical phenomenon.
In the broadest definition, a sensor is a device, module, machine, or subsystem that detects events or changes in its environment and sends ...
that can provide a range of optical and electronic responses.
The development of polythiophenes and related conductive organic polymers was recognized by the awarding of the 2000 Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
to Alan J. Heeger
Alan Jay Heeger (born January 22, 1936) is an American physicist, academic and Nobel Prize laureate in chemistry.
Heegar was elected as a member into the National Academy of Engineering in 2002 for co-founding the field of conducting polymers a ...
, Alan MacDiarmid
Alan Graham MacDiarmid, ONZ FRS (14 April 1927 – 7 February 2007) was a New Zealand-born American chemist, and one of three recipients of the Nobel Prize for Chemistry in 2000.
Early life and education
MacDiarmid was born in Masterton, New ...
, and Hideki Shirakawa "for the discovery and development of conductive polymers".
Mechanism of conductivity and doping
PT is an ordinary organic polymer, being a red solid that is poorly soluble in most solvents. Upon treatment with oxidizing agents (electron-acceptors) however, the material takes on a dark color and becomes electrically conductive. Oxidation is referred to as "doping". Around 0.2 equivalent of oxidant is used to convert PTs (and other conducting polymers) into the optimally conductive state. Thus about one of every five rings is oxidized. Many different oxidants are used. Because of the redox reaction, the conductive form of polythiophene is a salt. An idealized stoichiometry is shown using the oxidant F6: :(C4H2S)n + 1/5n F6 → (C4H2S)n(PF6)0.2n + 1/5 nA In principle, PT can be n-doped using reducing agents, but this approach is rarely practiced. Upon "p-doping", charged unit called a bipolaron is formed. The bipolaron moves as a unit along the polymer chain and is responsible for the macroscopically observed conductivity of the material. Conductivity can approach 1000 S/cm. In comparison, the conductivity ofcopper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
is approximately 5×105 S/cm. Generally, the conductivity of PTs is lower than 1000 S/cm, but high conductivity is not necessary for many applications, e.g. as an antistatic film.
Oxidants
A variety of reagents have been used to dope PTs.Iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
and bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...
produce highly conductive materials, which are unstable owing to slow evaporation of the halogen. Organic acid
An organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are ...
s, including trifluoroacetic acid
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF3CO2H. It is a structural analogue of acetic acid with all three of the acetyl group's hydrogen atoms replaced by fluorine atoms and is a colorless liquid with ...
, propionic acid
Propionic acid (, from the Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula CH3CH2CO2H. It is a li ...
, and sulfonic acids
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is kn ...
produce PTs with lower conductivities than iodine, but with higher environmental stabilities. Oxidative polymerization with ferric chloride
Iron(III) chloride is the inorganic compound with the formula . Also called ferric chloride, it is a common compound of iron in the +3 oxidation state. The anhydrous compound is a crystalline solid with a melting point of 307.6 °C. The col ...
can result in doping by residual catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, although matrix-assisted laser desorption/ionization
In mass spectrometry, matrix-assisted laser desorption/ionization (MALDI) is an ionization technique that uses a laser energy absorbing matrix to create ions from large molecules with minimal fragmentation. It has been applied to the analysis of ...
mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
(MALDI-MS) studies have shown that poly(3-hexylthiophene)s are also partially halogenated by the residual oxidizing agent. Poly(3-octylthiophene) dissolved in toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) ...
can be doped by solutions of ferric chloride hexahydrate dissolved in acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile ( hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
, and can be cast into films with conductivities reaching 1 S/cm. Other, less common p-dopants include gold trichloride and trifluoromethanesulfonic acid
Triflic acid, the short name for trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest known acids. Triflic acid is mainly used in research as a catalyst for es ...
.
Structure and optical properties
Conjugation length
The extended π-systems of conjugated PTs produce some of the most interesting properties of these materials—their optical properties. As an approximation, the conjugated backbone can be considered as a real-world example of the "electron-in-a-box" solution to theSchrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
; however, the development of refined models to accurately predict absorption
Absorption may refer to:
Chemistry and biology
*Absorption (biology), digestion
**Absorption (small intestine)
*Absorption (chemistry), diffusion of particles of gas or liquid into liquid or solid materials
*Absorption (skin), a route by which s ...
and fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, tha ...
spectra of well-defined oligo(thiophene) systems is ongoing. Conjugation relies upon overlap of the π-orbitals of the aromatic rings, which, in turn, requires the thiophene rings to be coplanar. The number of coplanar rings determines the conjugation length—the longer the conjugation length, the lower the separation between adjacent energy level
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The t ...
s, and the longer the absorption wavelength. Deviation from coplanarity may be permanent, resulting from mislinkages during synthesis or especially bulky side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a ...
s; or temporary, resulting from changes in the environment or binding. This twist in the backbone reduces the conjugation length, and the separation between energy levels is increased. This results in a shorter absorption wavelength.
Determining the maximum effective conjugation length requires the synthesis of regioregular PTs of defined length. The absorption band in the visible region is increasingly red-shifted as the conjugation length increases, and the maximum effective conjugation length is calculated as the saturation point of the red-shift. Early studies by ten Hoeve ''et al.'' estimated that the effective conjugation extended over 11 repeat units, while later studies increased this estimate to 20 units. Using the absorbance and emission profile of discrete conjugated oligo(3-hexylthiophene)s prepared through polymerization and separation, Lawrence ''et al.'' determined the effective conjugation length of poly(3-hexylthiophene) to be 14 units. The effective conjugation length of polythiophene derivatives depend on the chemical structure of side chains, and thiophene backbones.
A variety of environmental factors can cause the conjugated backbone to twist, reducing the conjugation length and causing an absorption band shift, including solvent, temperature, application of an electric field, and dissolved ions. The absorption band of poly ( 3-thiophene acetic acid) in aqueous solutions of poly(vinyl alcohol) (PVA) shifts from 480 nm at pH 7 to 415 nm at pH 4. This is attributed to formation of a compact coil structure, which can form hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
s with PVA upon partial deprotonation of the acetic acid group.
Shifts in PT absorption bands due to changes in temperature result from a conformational transition from a coplanar, rodlike structure at lower temperatures to a nonplanar, coiled structure at elevated temperatures. For example, poly(3-(octyloxy)-4-methylthiophene) undergoes a color change from red–violet at 25 °C to pale yellow at 150 °C. An isosbestic point (a point where the absorbance curves at all temperatures overlap) indicates coexistence between two phases, which may exist on the same chain or on different chains. Not all thermochromic
Thermochromism is the property of substances to change color due to a change in temperature. A mood ring is an excellent example of this phenomenon, but thermochromism also has more practical uses, such as baby bottles which change to a differen ...
PTs exhibit an isosbestic point: highly regioregular poly(3-alkylthiophene)s (PATs) show a continuous blue-shift with increasing temperature if the side chains are short enough so that they do not melt and interconvert between crystalline and disordered phases at low temperatures.
Optical effects
The optical properties of PTs can be sensitive to many factors. PTs exhibit absorption shifts due to application of electric potentials (electrochromism), or to introduction of alkali ions (ionochromism). Soluble PATs exhibit both thermochromism and solvatochromism (see above) in chloroform and 2,5-dimethyltetrahydrofuran.Substituted polythiophenes
Polythiophene and its oxidized derivatives have poor processing properties. They are insoluble in ordinary solvents and do not melt readily. For example, doped unsubstituted PTs are only soluble only exotic solvents such asarsenic trifluoride
Arsenic trifluoride is a chemical compound of arsenic and fluorine with the chemical formula AsF3. It is a colorless liquid which reacts readily with water.
Preparation and properties
It can be prepared by reacting hydrogen fluoride, HF, with ars ...
and arsenic pentafluoride. Although only poorly processable, "the expected high temperature stability and potentially very high electrical conductivity of PT films (if made) still make it a highly desirable material." Nonetheless, intense interest has focused on soluble polythiophenes, which usually translates to polymers derived from 3-alkylthiophenes, which give the so-called polyalkylthiophenes (PATs).
3-Alkylthiophenes
Soluble polymers are derivable from 3-substituted thiophenes where the 3-substituent is butyl or longer. Copolymers also are soluble, e.g., poly(3-methylthiophene-'co'-3'-octylthiophene).microstructure
Microstructure is the very small scale structure of a material, defined as the structure of a prepared surface of material as revealed by an optical microscope above 25× magnification. The microstructure of a material (such as metals, polymers ...
at the dyad level, 3-substituted thiophenes can couple to give any of three dyads:
*2,5', or head–tail (HT), coupling
*2,2', or head–head (HH), coupling
*5,5', or tail–tail (TT), coupling
These three diads can be combined into four distinct triads. The triads are distinguishable by NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique to observe local magnetic fields around atomic nuclei. The sample is placed in a magnetic fi ...
.
Regioregularity affects the properties of PTs. A regiorandom copolymer of 3-methylthiophene and 3-butylthiophene possessed a conductivity of 50 S/cm, whereas a more regioregular copolymer with a 2:1 ratio of HT to HH couplings had a higher conductivity of 140 S/cm. Films of regioregular poly(3-(4-octylphenyl)thiophene) (POPT) with greater than 94% HT content possessed conductivities of 4 S/cm, compared with 0.4 S/cm for regioirregular POPT. PATs prepared using Rieke zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
formed "crystalline, flexible, and bronze-colored films with a metallic luster". On the other hand, the corresponding regiorandom polymers produced "amorphous and orange-colored films". Comparison of the thermochromic properties of the Rieke PATs showed that, while the regioregular polymers showed strong thermochromic effects, the absorbance spectra of the regioirregular polymers did not change significantly at elevated temperatures. Finally, Fluorescence absorption and emission maxima of poly(3-hexylthiophene)s occur at increasingly lower wavelengths (higher energy) with increasing HH dyad content. The difference between absorption and emission maxima, the Stokes shift
__NOTOC__
Stokes shift is the difference (in energy, wavenumber or frequency units) between positions of the band maxima of the absorption and emission spectra ( fluorescence and Raman being two examples) of the same electronic transition. I ...
, also increases with HH dyad content, which they attributed to greater relief from conformational strain in the first excited state.
Special substituents
Water-soluble PT's are represented by sodium poly(3-thiophenealkanesulfonate)s. In addition to conferring water solubility, the pendant sulfonate groups act as counterions, producing self-doped conducting polymers. Substituted PTs with tetheredcarboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyli ...
s also exhibit water solubility. and urethanes
Thiophenes with chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
substituents at the 3 position have been polymerized. Such chiral PTs in principle could be employed for detection or separation of chiral analytes.
Poly(3-(perfluorooctyl)thiophene)s is soluble in supercritical carbon dioxide
Supercritical carbon dioxide (s) is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure.
Carbon dioxide usually behaves as a gas in air at standard temperature and pressure (STP), or as ...
Oligothiophenes capped at both ends with thermally-labile alkyl esters were cast as films from solution, and then heated to remove the solublizing end groups. Atomic force microscopy
Atomic force microscopy (AFM) or scanning force microscopy (SFM) is a very-high-resolution type of scanning probe microscopy (SPM), with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the opt ...
(AFM) images showed a significant increase in long-range order after heating.
Fluorinated polythiophene yield 7% efficiency in polymer-fullerene solar cells.
PEDOT
The 3,4-disubstituted thiophene called ethylenedioxythiophene (EDOT) is the precursor to the polymerPEDOT
Poly(3,4-ethylenedioxythiophene) (PEDOT or PEDT; ''IUPAC'' name poly(2,3-dihydrothieno ,4-''b''1,4]dioxane-5,7-diyl)) is a conducting polymer based on 3,4-ethylenedioxythiophene or EDOT. It was first reported by Bayer AG in 1989.
Polymer
PEDOT p ...
. Regiochemistry is not an issue in since this monomer is symmetrical. PEDOT is found in electrochromism, electrochromic displays, photovoltaic
Photovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect, a phenomenon studied in physics, photochemistry, and electrochemistry. The photovoltaic effect is commercially ...
s, electroluminescent displays, printed wiring, and sensors.
Synthesis
Electrochemical synthesis
In an electrochemical polymerization, a solution containing thiophene and anelectrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon ...
produces a conductive PT film on the anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ...
. Electrochemical polymerization is convenient, since the polymer does not need to be isolated and purified, but it can produce polymers with undesirable alpha-beta linkages and varying degrees of regioregularity. The stoichiometry of the electropolymerization is:
:n C4H4S → (C4H2S)n + 2n H+ + 2n e−
degree of polymerization
The degree of polymerization, or DP, is the number of monomeric units in a macromolecule or polymer or oligomer molecule.
For a homopolymer, there is only one type of monomeric unit and the ''number-average'' degree of polymerization is given by ...
and quality of the resulting polymer depends upon the electrode material, current density, temperature, solvent, electrolyte, presence of water, and monomer concentration.
Electron-donating substituents lower the oxidation potential, whereas electron-withdrawing groups increase the oxidation potential. Thus, 3-methylthiophene polymerizes in acetonitrile and tetrabutylammonium tetrafluoroborate at a potential of about 1.5 V vs. SCE, whereas unsubstituted thiophene requires an additional 0.2 V. Steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
resulting from branching at the α-carbon of a 3-substituted thiophene inhibits polymerization.
In terms of mechanism, oxidation of the thiophene monomer produces a radical cation
In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a ...
, which then couple with another monomer to produce a radical cation dimer.
From bromothiophenes
Chemical synthesis offers two advantages compared with electrochemical synthesis of PTs: a greater selection of monomers, and, using the proper catalysts, the ability to synthesize perfectly regioregular substituted PTs. PTs were chemically synthesized by accident more than a century ago. Chemical syntheses from 2,5-dibromothiophene use Kumada coupling and related reactions Regioregular PTs have been prepared by lithiation 2-bromo-3-alkylthiophenes using Kumada cross-coupling. This method produces approximately 100% HT–HT couplings, according to NMR spectroscopy analysis of the diads. 2,5-Dibromo-3-alkylthiophene when treated with highly reactive "Rieke zinc" is an alternative method.Routes employing chemical oxidants
In contrast to methods that require brominated monomers, the oxidative polymerization of thiophenes using ferric chloride proceeds at room temperature. The approach was reported by Sugimoto ''et al.'' in 1986. The stoichiometry is analogous to that of electropolymerization. This method has proven to be extremely popular; antistatic coatings are prepared on a commercial scale using ferric chloride. In addition to ferric chloride, other oxidizing agents have been reported. Slow addition of ferric chloride to the monomer solution produced poly(3-(4-octylphenyl)thiophene)s with approximately 94% H–T content. Precipitation of ferric chloride in situ (in order to maximize the surface area of the catalyst) produced significantly higher yields and monomer conversions than adding monomer directly to crystalline catalyst. Higher molecular weights were reported when dry air was bubbled through the reaction mixture during polymerization. Exhaustive Soxhlet extraction after polymerization with polar solvents was found to effectively fractionate the polymer and remove residual catalyst before NMR spectroscopy. Using a lower ratio of catalyst to monomer (2:1, rather than 4:1) may increase the regioregularity of poly(3-dodecylthiophene)s. Andreani ''et al.'' reported higher yields of soluble poly(dialkylterthiophene)s incarbon tetrachloride
Carbon tetrachloride, also known by many other names (such as tetrachloromethane, also recognised by the IUPAC, carbon tet in the cleaning industry, Halon-104 in firefighting, and Refrigerant-10 in HVACR) is an organic compound with the chemi ...
rather than chloroform, which they attributed to the stability of the radical species in carbon tetrachloride. Higher-quality catalyst, added at a slower rate and at reduced temperature, was shown to produce high molecular weight PATs with no insoluble polymer residue. Factorial experiments indicate that the catalyst/monomer ratio correlated with increased yield of poly(3-octylthiophene). Longer polymerization time also increased the yield.
toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) ...
, carbon tetrachloride, pentane
Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the ...
, and hexane
Hexane () is an organic compound, a straight-chain alkane with six carbon atoms and has the molecular formula C6H14.
It is a colorless liquid, odorless when pure, and with boiling points approximately . It is widely used as a cheap, relative ...
, and not diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable li ...
, xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are s ...
, acetone, or formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Est ...
), and speculated that the polymerization may occur at the surface of solid ferric chloride. However, this is challenged by the fact that the reaction also proceeds in acetonitrile, which FeCl3 is soluble in. Quantum mechanical calculations also point to a radical mechanism. The mechanism can also be inferred from the regiochemistry of the dimerization of 3-methylthiophene since C2 in -methylthiophenesup>+ has the highest spin density.
A carbocation mechanism is inferred from the structure of 3-(4-octylphenyl)thiophene prepared from ferric chloride.
Polymerization of thiophene can be effected by a solution of ferric chloride in acetonitrile. The kinetics of thiophene polymerization also seemed to contradict the predictions of the radical polymerization mechanism. Barbarella ''et al.'' studied the oligomerization of 3-(alkylsulfanyl)thiophenes, and concluded from their quantum mechanical calculations, and considerations of the enhanced stability of the radical cation when delocalized over a planar conjugated oligomer, that a radical cation mechanism analogous to that generally accepted for electrochemical polymerization was more likely. Given the difficulties of studying a system with a heterogeneous, strongly oxidizing catalyst that produces difficult to characterize rigid-rod polymers, the mechanism of oxidative polymerization is by no means decided. The radical cation mechanism is generally accepted.
Applications
 As an example of a static application, poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate) (PEDOT-PSS) product ("Clevios P") from
As an example of a static application, poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate) (PEDOT-PSS) product ("Clevios P") from Heraeus
Heraeus is a German technology group with a focus on precious and special metals, medical technology, quartz glass, sensors and specialty light sources. Founded in Hanau in 1851, the company is one of the largest family-owned companies in German ...
has been extensively used as an antistatic coating (as packaging materials for electronic components, for example). AGFA coats 200 m × 10 m of photographic film
Photographic film is a strip or sheet of transparent film base coated on one side with a gelatin emulsion containing microscopically small light-sensitive silver halide crystals. The sizes and other characteristics of the crystals determine ...
per year with PEDOT:PSS because of its antistatic
An antistatic agent is a compound used for treatment of materials or their surfaces in order to reduce or eliminate buildup of static electricity. Static charge may be generated by the triboelectric effect or by a non-contact process using a high ...
properties. The thin layer of PEDOT:PSS is virtually transparent and colorless, prevents electrostatic discharges during film rewinding, and reduces dust buildup on the negatives after processing.
Proposed applications
PEDOT also has been proposed for dynamic applications where a potential is applied to a polymer film. PEDOT-coated windows and mirrors become opaque or reflective upon the application of an electric potential, a manifestation of its electrochromic properties. Widespread adoption of electrochromic windows promise significant savings inair conditioning
Air conditioning, often abbreviated as A/C or AC, is the process of removing heat from an enclosed space to achieve a more comfortable interior environment (sometimes referred to as 'comfort cooling') and in some cases also strictly controlling ...
costs.
Another potential application include field-effect transistors, electroluminescent devices, solar cell
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.
s, photochemical resists, nonlinear optic devices, batteries
Battery most often refers to:
* Electric battery, a device that provides electrical power
* Battery (crime), a crime involving unlawful physical contact
Battery may also refer to:
Energy source
*Automotive battery, a device to provide power t ...
, diode
A diode is a two-terminal electronic component that conducts current primarily in one direction (asymmetric conductance); it has low (ideally zero) resistance in one direction, and high (ideally infinite) resistance in the other.
A diod ...
s, and chemical sensor
A sensor is a device that produces an output signal for the purpose of sensing a physical phenomenon.
In the broadest definition, a sensor is a device, module, machine, or subsystem that detects events or changes in its environment and sends ...
s. In general, two categories of applications are proposed for conducting polymers. Static applications rely upon the intrinsic conductivity of the materials, combined with their processing and material properties common to polymeric materials. Dynamic applications utilize changes in the conductive and optical properties, resulting either from application of electric potentials or from environmental stimuli.
PTs have been touted as sensor elements. In addition to biosensor applications, PTs can also be functionalized with receptors for detecting metal ions or chiral molecules as well. PTs with pendant crown ether functionalities exhibit properties that vary with the alkali metal. and main-chain.
Polythiophenes show potential in the treatment of prion diseases.
Further reading
*''Handbook of Conducting Polymers'' (Eds: T. A. Skotheim, R. L. Elsenbaumer, J. R. Reynolds), Marcel Dekker, New York, 1998. *G. Schopf, G. Koßmehl, ''Polythiophenes: Electrically Conductive Polymers'', Springer, Berlin, 1997, ;''Synthetic Metals''
(journal). ISSN 0379-6779 * * * *
References
{{reflist, 35em Molecular electronics Thiophenes Organic polymers Plastics Organic semiconductors Conductive polymers