Polysulfide on:
[Wikipedia]
[Google]
[Amazon]
 Polysulfides are a class of
Polysulfides are a class of
 Many commercial
Many commercial
 Polysulfides are a class of
Polysulfides are a class of chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s containing chains of sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
atoms. There are two main classes of polysulfides: inorganic and organic. Among the inorganic polysulfides, there are ones which contain anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s, which have the general formula . These anions are the conjugate bases of the hydrogen polysulfides . Organic polysulfides generally have the formulae , where R = alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
.
Polysulfide salts and complexes
The alkali metal polysulfides arise by treatment of a solution ofsulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds la ...
, e.g. sodium sulfide
Sodium sulfide is a chemical compound with the formula Na2 S, or more commonly its hydrate Na2S·9 H2O. Both the anhydrous and the hydrated salts in pure crystalline form are colorless solids, although technical grades of sodium sulfide are gene ...
, with elemental sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
:
:
In some cases, these anions have been obtained as organic salts, which are soluble in organic solvents.
The energy released in the reaction of sodium and elemental sulfur is the basis of battery technology. The sodium–sulfur battery and the lithium–sulfur battery require high temperatures to maintain liquid polysulfide and -conductive membranes that are unreactive toward sodium, sulfur, and sodium sulfide.
Polysulfides are ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
s in coordination chemistry
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Man ...
. Examples of transition metal polysulfido complexes include , , and . Main group elements also form polysulfides.
Organic polysulfides
In commerce, the term "polysulfide" usually refers to a class of polymers with alternating chains of several sulfur atoms and hydrocarbons. They have the formula . In this formula ''n'' indicates the number of sulfur atoms (or "rank"). Polysulfide polymers can be synthesized bycondensation polymerization
In polymer chemistry, condensation polymers are any kind of polymers whose process of polymerization involves a condensation reaction (i.e. a small molecule, such as water or methanol, is produced as a byproduct). Condensation polymers are ...
reactions between organic dihalides and alkali metal salts of polysulfide anions:
:
Dihalides used in this condensation polymerization are dichloroalkanes (such as 1,2-dichloroethane
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vin ...
, bis-(2-chloroethyl)formal (), and 1,3-dichloropropane). The polymers are called thiokols. In some cases, polysulfide polymers can be formed by ring-opening polymerization
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer (see figure). The reactive center can be radical, anio ...
reactions.
Polysulfide polymers are also prepared by the addition of polysulfanes to alkenes. An idealized equation is:
:
In reality, homogeneous samples of are difficult to prepare.
Polysulfide polymers are insoluble in water, oils, and many other organic solvents. Because of their solvent resistance, these materials find use as sealant
Sealant is a substance used to block the passage of fluids through openings in materials, a type of mechanical seal. In building construction ''sealant'' is sometimes synonymous with '' caulking'' and also serve the purposes of blocking dust, so ...
s to fill the joints in pavement, automotive window glass, and aircraft structures.
Polymers containing one or two sulfur atoms separated by hydrocarbon sequences are usually not classified polysulfides, e.g. poly(''p''-phenylene) sulfide .
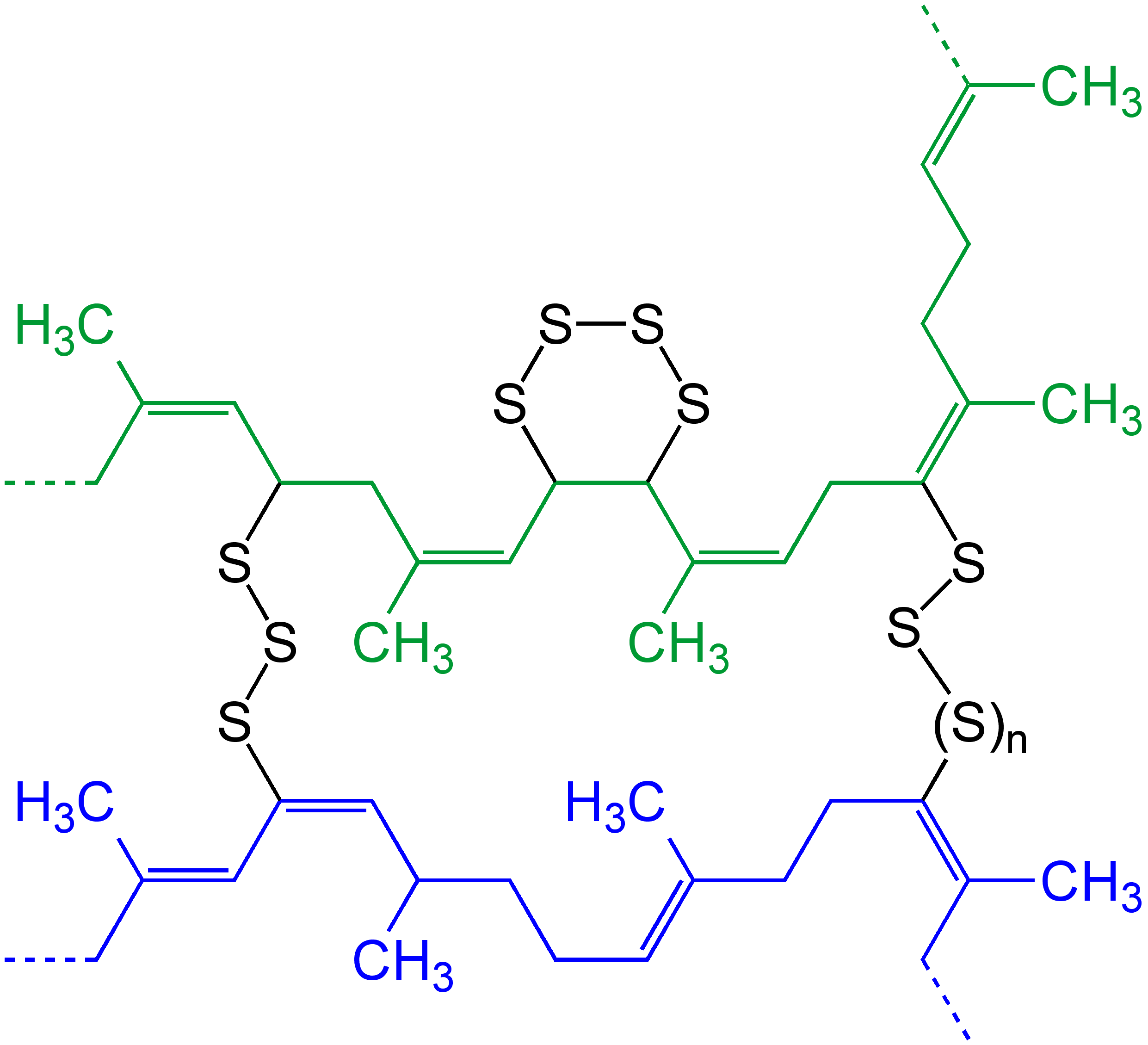
Polysulfides in vulcanized rubber
 Many commercial
Many commercial elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and Elasticity (physics), elasticity) and with weak intermolecular forces, generally low Young's modulus and high Deformation (mechanics), failure strain compared with other mate ...
s contain polysulfides as crosslinks. These crosslinks interconnect neighboring polymer chains, thereby conferring rigidity. The degree of rigidity is related to the number of crosslinks. Elastomers, therefore, have a characteristic ability to "snap back" to their original shape after being stretched or compressed. Because of this memory for their original cured shape, elastomers are commonly referred to as rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and ...
s. The process of crosslinking the polymer chains in these polymers with sulfur is called vulcanization
Vulcanization (British: Vulcanisation) is a range of processes for hardening rubbers. The term originally referred exclusively to the treatment of natural rubber with sulfur, which remains the most common practice. It has also grown to includ ...
. The sulfur chains attach themselves to the "allylic" carbon atoms, which are adjacent to C=C linkages. Vulcanization is a step in the processing of several classes of rubbers, including poly chloroprene (Neoprene
Neoprene (also polychloroprene) is a family of synthetic rubbers that are produced by polymerization of chloroprene.Werner Obrecht, Jean-Pierre Lambert, Michael Happ, Christiane Oppenheimer-Stix, John Dunn and Ralf Krüger "Rubber, 4. Emulsion R ...
), styrene-butadiene, and polyisoprene
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. Isoprene is an unsaturated hydrocarbon. It is produced by many plants and animals ...
, which is chemically similar to natural rubber. Charles Goodyear's discovery of vulcanization, involving the heating of polyisoprene with sulfur, was revolutionary because it converted a sticky and almost useless material into an elastomer that could be fabricated into useful products.
Occurrence in gas giants
In addition towater
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
, the clouds in the atmospheres of the gas giant
A gas giant is a giant planet composed mainly of hydrogen and helium. Gas giants are also called failed stars because they contain the same basic elements as a star. Jupiter and Saturn are the gas giants of the Solar System. The term "gas giant" ...
planets contain ammonium sulfides. The reddish-brownish clouds are attributed to polysulfides, arising from the exposure of the ammonium sulfides to light.
Properties
Polysulfides, as sulfides, can inducestress corrosion cracking
Stress corrosion cracking (SCC) is the growth of crack formation in a corrosive environment. It can lead to unexpected and sudden failure of normally ductile metal alloys subjected to a tensile stress, especially at elevated temperature. S ...
in carbon steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
* no minimum content is specified or required for chromium, coba ...
and stainless steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's r ...
.
See also
* * * *References
{{reflist Sulfur compounds Anions Inorganic polymers Corrosion Polysulfides