photoanode on:
[Wikipedia]
[Google]
[Amazon]
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer,
H2O(l) + v+ 2h+ -> 2H+ (aq) + 1/2O2(g)
This leaves positive charge carriers (protons, that is, H+ ions) in solution, which must then bond with one other proton and combine with two electrons in order to form hydrogen gas, according to:
:::::::::::::2H+ + 2e- -> H2(g)
A photosynthetic cell is another form of photoelectrolytic cell, with the output in that case being carbohydrates instead of molecular hydrogen.
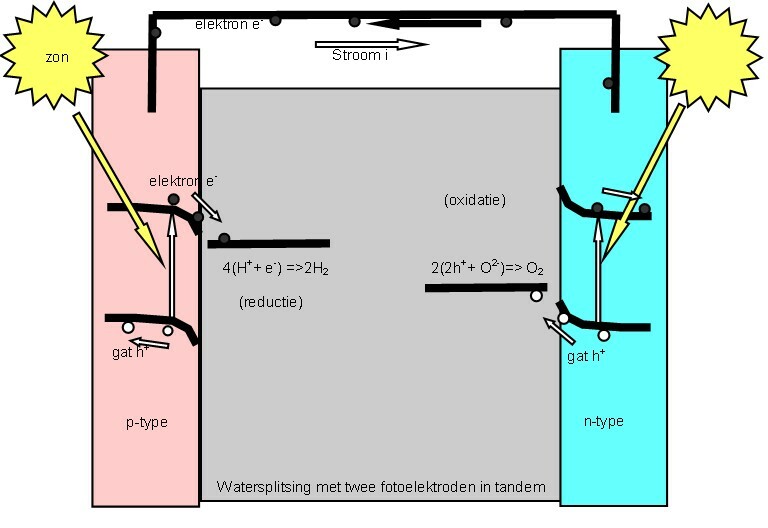 A (water-splitting) photoelectrolytic cell electrolizes water into
A (water-splitting) photoelectrolytic cell electrolizes water into
H2 production:
* light absorbance: determined by band gap and appropriate for solar irradiation spectrum
* charge transport: photoelectrodes must be conductive (or semi-conductive) to minimize resistive losses
* suitable band structure: large enough band gap to split water (1.23V) and appropriate positions relative to redox potentials for H2 and O2
* catalytic activity: high catalytic activity increases efficiency of the water-splitting reaction
* stability: materials must be stable to prevent decomposition and loss of function
In addition to these requirements, materials must be low-cost and earth abundant for the widespread adoption of PEC water splitting to be feasible.
While the listed requirements can be applied generally, photoanodes and photocathodes have slightly different needs. A good photocathode will have early onset of the oxygen evolution reaction (low overpotential), a large photocurrent at saturation, and rapid growth of photocurrent upon onset. Good photoanodes, on the other hand, will have early onset of the hydrogen evolution reaction in addition to high current and rapid photocurrent growth. To maximize current, anode and cathode materials need to be matched together; the best anode for one cathode material may not be the best for another.
 Researchers have extensively investigated the use of hematite (α-Fe2O3) in PEC water-splitting devices due to its low cost, ability to be n-type doped, and band gap (2.2eV). However, performance is plagued by poor conductivity and crystal anisotropy. Some researchers have enhanced catalytic activity by forming a layer of co-catalysts on the surface. Co-catalysts include cobalt-phosphate and iridium oxide, which is known to be a highly active catalyst for the oxygen evolution reaction.
Researchers have extensively investigated the use of hematite (α-Fe2O3) in PEC water-splitting devices due to its low cost, ability to be n-type doped, and band gap (2.2eV). However, performance is plagued by poor conductivity and crystal anisotropy. Some researchers have enhanced catalytic activity by forming a layer of co-catalysts on the surface. Co-catalysts include cobalt-phosphate and iridium oxide, which is known to be a highly active catalyst for the oxygen evolution reaction.
H2 generation from seawater, which is much more difficult due to the presence of contaminating ions and a more harsh corrosive environment.
EERE-Photoelectrochemical Generation of Hydrogen Using Heterostructural Titania Nanotube ArraysMano
{{Fuel cells Materials science Energy conversion Photochemistry Hydrogen production Fuel cells Solar cells Photoelectrochemistry
semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way ...
, or aqueous metal
A metal (from ancient Greek, Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, e ...
immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
via the electrolysis of water.
Both types of device are varieties of solar cell
A solar cell, or photovoltaic cell, is an electronic device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon.
, in that a photoelectrochemical cell's function is to use the photoelectric effect (or, very similarly, the photovoltaic effect) to convert electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visib ...
(typically sunlight) either directly into electrical power, or into something which can itself be easily used to produce electrical power (hydrogen, for example, can be burned to create electrical power, see photohydrogen).
Two principles
The standard photovoltaic effect, as operating in standard photovoltaic cells, involves the excitation of negative charge carriers (electrons) within a semiconductor medium, and it is negative charge carriers (free electrons) which are ultimately extracted to produce power. The classification of photoelectrochemical cells which includes Grätzel cells meets this narrow definition, albeit the charge carriers are oftenexciton
An exciton is a bound state of an electron and an electron hole which are attracted to each other by the electrostatic Coulomb force. It is an electrically neutral quasiparticle that exists in insulators, semiconductors and some liquids. ...
ic.
The situation within a photoelectrolytic cell, on the other hand, is quite different. For example, in a water-splitting photoelectrochemical cell, the excitation, by light, of an electron in a semiconductor leaves a hole which "draws" an electron from a neighboring water molecule:
:::::::::::::Photoelectrolytic cell
 A (water-splitting) photoelectrolytic cell electrolizes water into
A (water-splitting) photoelectrolytic cell electrolizes water into hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
gas by irradiating the anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ...
with electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visib ...
, that is, with light. This has been referred to as artificial photosynthesis
Artificial photosynthesis is a chemical process that biomimics the natural process of photosynthesis to convert sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term artificial photosynthesis is commonly used to refer ...
and has been suggested as a way of storing solar energy
Solar energy is radiant light and heat from the Sun that is harnessed using a range of technologies such as solar power to generate electricity, solar thermal energy (including solar water heating), and solar architecture. It is an essen ...
in hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
for use as fuel.
Incoming sunlight excites free electrons near the surface of the silicon electrode. These electrons flow through wires to the stainless steel electrode, where four of them react with four water molecules to form two molecules of hydrogen and 4 OH groups. The OH groups flow through the liquid electrolyte to the surface of the silicon electrode. There they react with the four holes associated with the four photoelectrons, the result being two water molecules and an oxygen molecule. Illuminated silicon immediately begins to corrode under contact with the electrolytes. The corrosion consumes material and disrupts the properties of the surfaces and interfaces within the cell.
Two types of photochemical systems operate via photocatalysis
In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalyzed photolysis, light is absorbed by an adsorbed substrate. In photogenerated catalysis, the photocatalytic activity depends on the a ...
. One uses semiconductor surfaces as catalysts. In these devices the semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way ...
surface absorbs solar energy and acts as an electrode for water splitting
Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:
:2 H2O → 2 H2 + O2
Efficient and economical water splitting would be a technological breakthrough that could underpin a hydrogen economy, base ...
. The other methodology uses in-solution metal complexes as catalysts.
Photoelectrolytic cells have passed the 10 percent economic efficiency
In microeconomics, economic efficiency, depending on the context, is usually one of the following two related concepts:
* Allocative or Pareto efficiency: any changes made to assist one person would harm another.
* Productive efficiency: no addi ...
barrier. Corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
of the semiconductors
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way. ...
remains an issue, given their direct contact with water. Research is now ongoing to reach a service life
A product's service life is its period of use in service. Several related terms describe more precisely a product's life, from the point of manufacture, storage, and distribution, and eventual use.
Service life has been defined as "a product's ...
of 10000 hours, a requirement established by the United States Department of Energy
The United States Department of Energy (DOE) is an executive department of the U.S. federal government that oversees U.S. national energy policy and manages the research and development of nuclear power and nuclear weapons in the United States ...
.
Other photoelectrochemical cells
The first photovoltaic cell ever designed was also the first photoelectrochemical cell. It was created in 1839, by Alexandre-Edmond Becquerel, at age 19, in his father's laboratory. The mostly commonly researched modern photoelectrochemical cell in recent decades has been theGrätzel cell
A dye-sensitized solar cell (DSSC, DSC, DYSC or Grätzel cell) is a low-cost solar cell belonging to the group of thin film solar cells. It is based on a semiconductor formed between a photo-sensitized anode and an electrolyte, a '' photoele ...
, although much attention has recently shifted away from this topic to perovskite solar cells, due to relatively high efficiency of the latter and the similarity in vapor assisted deposition techniques commonly used in their creation.
Dye-sensitized solar cells or Grätzel cells use dye- adsorbed highly porous nanocrystalline titanium dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insolu ...
(nc-) to produce electrical energy.
Materials for photoelectrolytic cells
Water-splitting photoelectrochemical (PEC) cells use light energy to decompose water into hydrogen and oxygen within a two-electrode cell. In theory, three arrangements of photo-electrodes in the assembly of PECs exist: * photo-anode made of a n-type semiconductor and a metal cathode * photo-anode made of a n-type semiconductor and a photo-cathode made of a p-type semiconductor * photo-cathode made of a p-type semiconductor and a metal anode There are several requirements for photoelectrode materials in PECIn 1967, Akira Fujishima discovered the Honda-Fujishima effect, (the photocatalytic properties of titanium dioxide). and other metal oxides are still most prominent catalysts for efficiency reasons. Including and , this kind of semiconducting titanates, the
conduction band
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level, and thus determine the electrical conductivity of the solid. In nonmetals, the valence band is the highest range of electron energies in ...
has mainly titanium 3d character and the valence band oxygen 2p character. The bands are separated by a wide band gap of at least 3 eV, so that these materials absorb only UV radiation
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiatio ...
.
Change of the microstructure has also been investigated to further improve the performance. In 2002, Guerra (Nanoptek Corporation) discovered that high localized strain could be induced in semiconductor films formed on micro to nano-structured templates, and that this strain shifted the bandgap of the semiconductor, in the case of titanium dioxide, into the visible blue. It was further found (Thulin and Guerra, 2008) that the strain also favorably shifted the band-edges to overlay the hydrogen evolution potential, and further still that the strain improved hole mobility, for lower charge recombination rate and high quantum efficiency. Chandekar developed a low-cost scalable manufacturing process to produce both the nano-structured template and the strained titanium dioxide coating. Other morphological investigations include nanowire arrays or porous nanocrystalline photoelectrochemical cells.
GaN
GaN is another option, because metal nitrides usually have a narrow band gap that could encompass almost the entire solar spectrum. GaN has a narrower band gap than but is still large enough to allow water splitting to occur at the surface. GaN nanowires exhibited better performance than GaN thin films, because they have a larger surface area and have a high single crystallinity which allows longer electron-hole pair lifetimes. Meanwhile, other non-oxide semiconductors such asGaAs
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a zinc blende crystal structure.
Gallium arsenide is used in the manufacture of devices such as microwave frequency integrated circuits, monolithic microwave integrat ...
, , and are used as n-type electrode, due to their stability in chemical and electrochemical steps in the photocorrosion reactions.
Silicon
In 2013 a cell with 2 nanometers of nickel on a silicon electrode, paired with a stainless steel electrode, immersed in an aqueous electrolyte of potassium borate and lithium borate operated for 80 hours without noticeable corrosion, versus 8 hours for titanium dioxide. In the process, about 150 ml of hydrogen gas was generated, representing the storage of about 2 kilojoules of energy.Structured materials
Structuring of absorbing materials has both positive and negative affects on cell performance. Structuring allows for light absorption and carrier collection to occur in different places, which loosens the requirements for pure materials and helps with catalysis. This allows for the use of non-precious and oxide catalysts that may be stable in more oxidizing conditions. However, these devices have lower open-circuit potentials which may contribute to lower performance.Hematite
 Researchers have extensively investigated the use of hematite (α-Fe2O3) in PEC water-splitting devices due to its low cost, ability to be n-type doped, and band gap (2.2eV). However, performance is plagued by poor conductivity and crystal anisotropy. Some researchers have enhanced catalytic activity by forming a layer of co-catalysts on the surface. Co-catalysts include cobalt-phosphate and iridium oxide, which is known to be a highly active catalyst for the oxygen evolution reaction.
Researchers have extensively investigated the use of hematite (α-Fe2O3) in PEC water-splitting devices due to its low cost, ability to be n-type doped, and band gap (2.2eV). However, performance is plagued by poor conductivity and crystal anisotropy. Some researchers have enhanced catalytic activity by forming a layer of co-catalysts on the surface. Co-catalysts include cobalt-phosphate and iridium oxide, which is known to be a highly active catalyst for the oxygen evolution reaction.
Tungsten oxide
Tungsten(VI) oxide
Tungsten(VI) oxide, also known as tungsten trioxide is a chemical compound of oxygen and the transition metal tungsten, with formula WO3. The compound is also called tungstic anhydride, reflecting its relation to tungstic acid . It is a ligh ...
(WO3), which exhibits several different polymorphs at various temperatures, is of interest due to its high conductivity but has a relatively wide, indirect band gap (~2.7 eV) which means it cannot absorb most of the solar spectrum. Though many attempts have been made to increase absorption, they result in poor conductivity and thus WO3 does not appear to be a viable material for PEC water splitting.
Bismuth vanadate
With a narrower, direct band gap (2.4 eV) and proper band alignment with water oxidation potential, the monoclinic form of has garnered interest from researchers. Over time, it has been shown that V-rich and compact films are associated with higher photocurrent, or higher performance. Bismuth Vanadate has also been studied for solarOxidation form
Photoelectrochemical oxidation (PECO) is the process by which light enables asemiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way ...
to promote a catalytic oxidation
Catalytic oxidation are processes that rely on catalysts to introduce oxygen into organic and inorganic compounds. Many applications, including the focus of this article, involve oxidation by oxygen. Such processes are conducted on a large scale ...
reaction. While a photoelectrochemical cell typically involves both a semiconductor (electrode) and a metal (counter-electrode), at sufficiently small scales, pure semiconductor particles can behave as microscopic photoelectrochemical cells. PECO has applications in the detoxification of air and water, hydrogen production, and other applications.
Reaction mechanism
The process by which a photon initiates a chemical reaction directly is known asphotolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
; if this process is aided by a catalyst, it is called photocatalysis
In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalyzed photolysis, light is absorbed by an adsorbed substrate. In photogenerated catalysis, the photocatalytic activity depends on the a ...
.D. Y. Goswami, Principles of solar engineering, 3rd ed. Boca Raton: Taylor & Francis, 2015. If a photon has more energy than a material's characteristic band gap, it can free an electron upon absorption by the material. The remaining, positively charged hole and the free electron may recombine, generating heat, or they can take part in photoreactions with nearby species. If the photoreactions with these species result in regeneration of the electron-donating material—i.e., if the material acts as a catalyst for the reactions—then the reactions are deemed photocatalytic. PECO represents a type of photocatalysis whereby semiconductor-based electrochemistry catalyzes an oxidation reaction—for example, the oxidative degradation of an airborne contaminant in air purification systems.
The principal objective of photoelectrocatalysis is to provide low-energy activation pathways for the passage of electronic charge carriers through the electrode electrolyte interface and, in particular, for the photoelectrochemical generation of chemical products.H. Tributsch, "Photoelectrocatalysis," in Photocatalysis: Fundamentals and Applications, N. Serpone and E. Pelizzetti, Eds., ed New York: Wiley-Interscience, 1989, pp. 339-383. With regard to photoelectrochemical oxidation, we may consider, for example, the following system of reactions, which constitute TiO2-catalyzed oxidation.O. Legrini, E. Oliveros, and A. Braun, "Photochemical processes for water treatment," Chemical Reviews, vol. 93, pp. 671-698, 1993.
:TiO2 (hv) → TiO2 (e− + h+)
:TiO2(h+) +RX → TiO2 + RX.+
:TiO2(h+) + H2O → TiO2 + HO. + H+
:TiO2(h+) + OH− → TiO2 + HO.
:TiO2(e−) + O2 → TiO2 + O2.−
This system shows a number of pathways for the production of oxidative species that facilitate the oxidation of the species, RX, in addition to its direct oxidation by the excited TiO2 itself. PECO concerns such a process where the electronic charge carriers are able to readily move through the reaction medium, thereby to some extent mitigating recombination reactions that would limit the oxidative process. The “photoelectrochemical cell” in this case could be as simple as a very small particle of the semiconductor catalyst. Here, on the “light” side a species is oxidized, while on the “dark” side a separate species is reduced.D. Y. Goswami, "Photoelectrochemical air disinfection " US Patent 7,063,820 B2, 2006.
Photochemical oxidation (PCO) versus PECO
The classical macroscopic photoelectrochemical system consists of a semiconductor in electric contact with a counter-electrode. ForN-type semiconductor
An extrinsic semiconductor is one that has been '' doped''; during manufacture of the semiconductor crystal a trace element or chemical called a doping agent has been incorporated chemically into the crystal, for the purpose of giving it differen ...
particles of sufficiently small dimension, the particles polarize into anodic and cathodic regions, effectively forming microscopic photoelectrochemical cells. The illuminated surface of a particle catalyzes a photooxidation
In polymer chemistry photo-oxidation (sometimes: oxidative photodegradation) is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidatio ...
reaction, while the “dark” side of the particle facilitates a concomitant reduction.
Photoelectrochemical oxidation may be thought of as a special case of photochemical oxidation (PCO). Photochemical oxidation entails the generation of radical species that enable oxidation reactions, with or without the electrochemical interactions involved in semiconductor-catalyzed systems, which occur in photoelectrochemical oxidation.
Applications
PECO may be useful in treating both air and water, as well as producing hydrogen as a source of renewable energy.Water Treatment
PECO has shown promise forwater treatment
Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, inc ...
of both stormwater
Stormwater, also spelled storm water, is water that originates from precipitation ( storm), including heavy rain and meltwater from hail and snow. Stormwater can soak into the soil ( infiltrate) and become groundwater, be stored on depressed l ...
and wastewater. Currently, water treatment methods like the use of biofiltration
Biofiltration is a pollution control technique using a bioreactor containing living material to capture and biologically degrade pollutants. Common uses include processing waste water, capturing harmful chemicals or silt from surface runoff, a ...
technologies are widely used. These technologies are effective at filtering out pollutants like suspended solids, nutrients, and heavy metals, but struggle to remove herbicides. Herbicides like diuron and atrazine
Atrazine is a chlorinated herbicide of the triazine class. It is used to prevent pre-emergence broadleaf weeds in crops such as maize (corn), soybean and sugarcane and on turf, such as golf courses and residential lawns. Atrazine's primary m ...
are commonly used, and often end up in stormwater, posing potential health risks if they are not treated before reuse.
PECO is a useful solution to treating stormwater because of its strong oxidation capacity. Investigating different mechanisms for herbicide degradation in stormwater, like PECO, photocatalytic oxidation (PCO), and electro-catalytic oxidation (ECO), researchers determined that PECO was the best option, demonstrating complete mineralization of diuron in one hour. Further research into this use for PECO is needed, as it was only able to degrade 35% of atrazine in that time, however it is a promising solution moving forward.
Air Treatment
PECO has also shown promise as a means ofair purification
An air purifier or air cleaner is a device which removes contaminants from the air in a room to improve indoor air quality. These devices are commonly marketed as being beneficial to allergy sufferers and asthmatics, and at reducing or eliminating ...
. For people with severe allergies, air purifiers are important to protect them from allergens within their own homes. However, some allergens are too small to be removed by normal purification methods. Air purifiers using PECO filters are able to remove particles as small as 0.1 nm.
These filters work as photons excite a photocatalyst, creating hydroxyl free radicals, which are extremely reactive and oxidize organic material and microorganisms that cause allergy symptoms, forming harmless products like carbon dioxide and water. Researchers testing this technology with patients suffering from allergies drew promising conclusions from their studies, observing significant reductions in total symptom scores (TSS) for both nasal (TNSS) and ocular (TOSS) allergies after just 4 weeks of using the PECO filter. This research demonstrates strong potential for impactful health improvements who suffer from severe allergies and asthma.
Hydrogen Production
Possibly the most exciting potential use for PECO is producing hydrogen to be used as a source ofrenewable energy
Renewable energy is energy that is collected from renewable resources that are naturally replenished on a human timescale. It includes sources such as sunlight, wind, the movement of water, and geothermal heat. Although most renewable energy ...
. Photoelectrochemical oxidation reactions that take place within PEC cells are the key to water splitting for hydrogen production. While the main concern with this technology is stability, systems that use PECO technology to create hydrogen from vapor rather than liquid water has demonstrated potential for greater stability. Early researchers working on vapor fed systems developed modules with 14% solar to hydrogen (STH) efficiency, while remaining stable for 1000+ hours. More recently, further technological developments have been made, demonstrated by the direct air electrolysis (DAE) module developed by Jining Guo and his team, which produces 99% pure hydrogen from the air and has demonstrated stability of 8 months thus far.
Promising research and technological advancement using PECO for different applications like water and air treatment and hydrogen production suggests that it is a valuable tool that can be utilized in a variety of ways.
History
In 1938, Goodeve and Kitchener demonstrated the “photosensitization” of TiO2—e.g., as evidenced by the fading of paints incorporating it as a pigment. In 1969, Kinney and Ivanuski suggested that a variety of metal oxides, including TiO2, may catalyze the oxidation of dissolved organic materials (phenol, benzoic acid, acetic acid, sodium stearate, and sucrose) under illumination by sunlamps.L. C. Kinney and V. R. Ivanuski, "Photolysis mechanisms for pollution abatement," 1969. Additional work by Carey et al. suggested that TiO2 may be useful for the photodechlorination of PCBs.J. H. Carey, J. Lawrence, and H. M. Tosine, "Photodechlorination of PCB's in the presence of titanium dioxide in aqueous suspensions," Bulletin of Environmental Contamination and Toxicology, vol. 16, pp. 697–701, 1976.Further reading
*I. U. I. A. Gurevich, I. U. V. Pleskov, and Z. A. Rotenberg, Photoelectrochemistry. New York: Consultants Bureau, 1980. *M. Schiavello, Photoelectrochemistry, photocatalysis, and photoreactors: Fundamentals and developments. Dordrecht: Reidel, 1985. *A. J. Bard, M. Stratmann, and S. Licht, Encyclopedia of Electrochemistry, Volume 6, Semiconductor Electrodes and Photoelectrochemistry: Wiley, 2002.See also
*Artificial photosynthesis
Artificial photosynthesis is a chemical process that biomimics the natural process of photosynthesis to convert sunlight, water, and carbon dioxide into carbohydrates and oxygen. The term artificial photosynthesis is commonly used to refer ...
* Glossary of fuel cell terms
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to ...
* Photoelectrolysis of water
* Photocatalytic water splitting
* Photochemical reaction Organic photochemistry encompasses organic reactions that are induced by the action of light. The absorption of ultraviolet light by organic molecules often leads to reactions. In the earliest days, sunlight was employed, while in more modern times ...
* Photochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet ( wavelength from 100 to 400 nm), visible light (400� ...
* Photodissociation
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
* Photoelectrochemistry
Photoelectrochemistry is a subfield of study within physical chemistry concerned with the interaction of light with electrochemical systems. It is an active domain of investigation. One of the pioneers of this field of electrochemistry was the Ge ...
* Photoelectrolysis
Photoelectrochemistry is a subfield of study within physical chemistry concerned with the interaction of light with electrochemical systems. It is an active domain of investigation. One of the pioneers of this field of electrochemistry was the Ge ...
* Photohydrogen
* Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in ...
* Timeline of hydrogen technologies
References
External links
EERE-Photoelectrochemical Generation of Hydrogen Using Heterostructural Titania Nanotube ArraysMano
{{Fuel cells Materials science Energy conversion Photochemistry Hydrogen production Fuel cells Solar cells Photoelectrochemistry