Phenol on:
[Wikipedia]
[Google]
[Amazon]
Phenol (also called carbolic acid) is an
C6H5OH <=> C6H5O- + H+
 Phenol is more acidic than aliphatic alcohols. The differing pKa is attributed to resonance stabilization of the phenoxide anion. In this way, the negative charge on oxygen is delocalized on to the ortho and para carbon atoms through the pi system. An alternative explanation involves the sigma framework, postulating that the dominant effect is the induction from the more electronegative sp2 hybridised carbons; the comparatively more powerful inductive withdrawal of electron density that is provided by the sp2 system compared to an sp3 system allows for great stabilization of the oxyanion. In support of the second explanation, the p''K''a of the
Phenol is more acidic than aliphatic alcohols. The differing pKa is attributed to resonance stabilization of the phenoxide anion. In this way, the negative charge on oxygen is delocalized on to the ortho and para carbon atoms through the pi system. An alternative explanation involves the sigma framework, postulating that the dominant effect is the induction from the more electronegative sp2 hybridised carbons; the comparatively more powerful inductive withdrawal of electron density that is provided by the sp2 system compared to an sp3 system allows for great stabilization of the oxyanion. In support of the second explanation, the p''K''a of the
 Phenol exhibits keto-enol tautomerism with its unstable keto tautomer cyclohexadienone, but only a tiny fraction of phenol exists as the keto form. The equilibrium constant for enolisation is approximately 10−13, which means only one in every ten trillion molecules is in the keto form at any moment. The small amount of stabilisation gained by exchanging a C=C bond for a C=O bond is more than offset by the large destabilisation resulting from the loss of aromaticity. Phenol therefore exists essentially entirely in the enol form. 4, 4' Substituted cyclohexadienone can undergo a
Phenol exhibits keto-enol tautomerism with its unstable keto tautomer cyclohexadienone, but only a tiny fraction of phenol exists as the keto form. The equilibrium constant for enolisation is approximately 10−13, which means only one in every ten trillion molecules is in the keto form at any moment. The small amount of stabilisation gained by exchanging a C=C bond for a C=O bond is more than offset by the large destabilisation resulting from the loss of aromaticity. Phenol therefore exists essentially entirely in the enol form. 4, 4' Substituted cyclohexadienone can undergo a
 Phenol is highly reactive toward electrophilic aromatic substitution. The enhance nucleophilicity is attributed to donation pi electron density from O into the ring. Many groups can be attached to the ring, via halogenation, acylation, sulfonation, and related processes. Phenol's ring is so strongly activated that bromination and chlorination lead readily to polysubstitution. Phenol reacts with dilute nitric acid at room temperature to give a mixture of 2-nitrophenol and 4-nitrophenol while with concentrated nitric acid, additional nitro groups are introduced, e.g. to give
Phenol is highly reactive toward electrophilic aromatic substitution. The enhance nucleophilicity is attributed to donation pi electron density from O into the ring. Many groups can be attached to the ring, via halogenation, acylation, sulfonation, and related processes. Phenol's ring is so strongly activated that bromination and chlorination lead readily to polysubstitution. Phenol reacts with dilute nitric acid at room temperature to give a mixture of 2-nitrophenol and 4-nitrophenol while with concentrated nitric acid, additional nitro groups are introduced, e.g. to give C6H5OH + NaOH -> C6H5ONa + H2O
When a mixture of phenol and benzoyl chloride are shaken in presence of dilute sodium hydroxide solution, phenyl benzoate is formed. This is an example of the Schotten–Baumann reaction:
:C6H5COCl + HOC6H5 -> C6H5CO2C6H5 + HCl
Phenol is reduced to benzene when it is distilled with C6H5OH + Zn -> C6H6 + ZnO
When phenol is treated with diazomethane in the presence of boron trifluoride (), anisole is obtained as the main product and nitrogen gas as a byproduct.
:C6H5OH + CH2N2 -> C6H5OCH3 + N2
When phenol reacts with iron(III) chloride solution, an intense violet-purple solution is formed.
 Accounting for 95% of production (2003) is the
Accounting for 95% of production (2003) is the
C6H6 + O -> C6H5OH
C6H5CH3 + 2 O2 -> C6H5OH + CO2 + H2O
The reaction is proposed to proceed via formation of benzyoylsalicylate.
C6H5SO3H + 2 NaOH -> C6H5OH + Na2SO3 + H2O
C6H5Cl + NaOH -> C6H5OH + NaCl
:C6H5Cl + H2O -> C6H5OH + HCl
These methods suffer from the cost of the chlorobenzene and the need to dispose of the chloride by product.
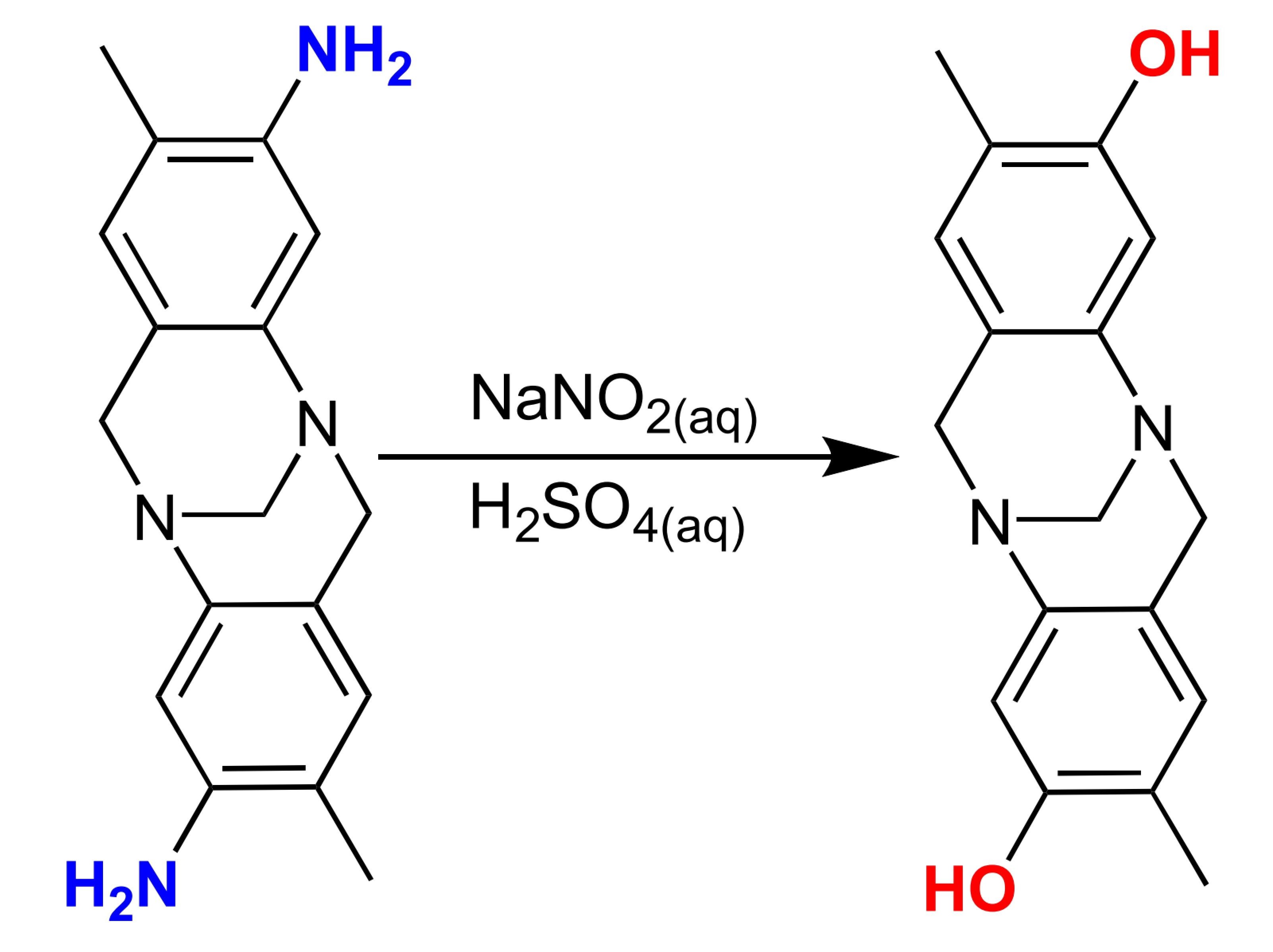
C6H5NH2 + HCl/NaNO2 -> C6H5OH + N2 + H2O + NaCl
: Salicylic acid decarboxylates to phenol.
Salicylic acid decarboxylates to phenol.
by Peter Tyson. NOVA It was originally used by the Nazis in 1939 as part of the Aktion T4 euthanasia program.''The Nazi Doctors''
, Chapter 14, Killing with Syringes: Phenol Injections. By Dr. Robert Jay Lifton The Germans learned that extermination of smaller groups was more economical by injection of each victim with phenol. Phenol injections were given to thousands of people.
International Chemical Safety Card 0070National Pollutant Inventory: Phenol Fact SheetCDC - Phenol - NIOSH Workplace Safety and Health Topic
{{Authority control Antiseptics Commodity chemicals Hazardous air pollutants Oxoacids Phenyl compounds 1834 in science
aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
with the molecular formula . It is a white crystalline solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structur ...
that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns.
Phenol was first extracted from coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat pso ...
, but today is produced on a large scale (about 7 billion kg/year) from petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
-derived feedstocks. It is an important industrial commodity as a precursor
Precursor or Precursors may refer to:
* Precursor (religion), a forerunner, predecessor
** The Precursor, John the Baptist
Science and technology
* Precursor (bird), a hypothesized genus of fossil birds that was composed of fossilized parts of u ...
to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies
The Epoxies were an American new wave band from Portland, Oregon, formed in 2000. Heavily influenced by new wave, the band jokingly described themselves as robot garage rock. Members included FM Static on synthesizers, guitarist Viz Spectrum, l ...
, Bakelite, nylon
Nylon is a generic designation for a family of synthetic polymers composed of polyamides ( repeating units linked by amide links).The polyamides may be aliphatic or semi-aromatic.
Nylon is a silk-like thermoplastic, generally made from pet ...
, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs.
Properties
Phenol is an organic compound appreciably soluble in water, with about 84.2 g dissolving in 1000 mL (0.895 M). Homogeneous mixtures of phenol and water at phenol to water mass ratios of ~2.6 and higher are possible. The sodium salt of phenol, sodium phenoxide, is far more water-soluble.Acidity
Phenol is a weak acid. In aqueous solution in the pH range ca. 8 - 12 it is in equilibrium with the phenolate anion (also called phenoxide): :enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). T ...
of acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscibl ...
in water is 10.9, making it only slightly less acidic than phenol (p''K''a 10.0). Thus, the greater number of resonance structures available to phenoxide compared to acetone enolate seems to contribute very little to its stabilization. However, the situation changes when solvation effects are excluded. A recent ''in silico'' comparison of the gas phase acidities of the vinylogues of phenol and cyclohexanol in conformations that allow for or exclude resonance stabilization leads to the inference that about of the increased acidity of phenol is attributable to inductive effects, with resonance accounting for the remaining difference.
Hydrogen bonding
In carbon tetrachloride and alkane solvents phenol hydrogen bonds with a wide range of Lewis bases such aspyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid w ...
, diethyl ether, and diethyl sulfide. The enthalpies of adduct formation and the IR frequency shifts accompanying adduct formation have been studied. Phenol is classified as a hard acid which is compatible with the ''C''/''E'' ratio of the ''ECW'' model with ''E''A = 2.27 and ''C''A = 1.07. The relative acceptor strength of phenol toward a series of bases, versus other Lewis acids, can be illustrated by C-B plots.
Phenoxide anion
The phenoxide anion is a strong nucleophile with anucleophilicity
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
comparable to the one of carbanions or tertiary amines. It can react at both its oxygen or carbon sites as an ambident nucleophile (see HSAB theory). Generally, oxygen attack of phenoxide anions is kinetically favored, while carbon-attack is thermodynamically preferred (see Thermodynamic versus kinetic reaction control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or ...
). Mixed oxygen/carbon attack and by this a loss of selectivity is usually observed if the reaction rate reaches diffusion control.
Tautomerism
dienone–phenol rearrangement
The dienone–phenol rearrangement is a reaction in organic chemistry first reported in 1921 by Karl von Auwers and Karl Ziegler. A common example of dienone–phenol rearrangement is 4,4-disubstituted converting into a stable 3,4-disubstituted ...
in acid conditions and form stable 3,4‐disubstituted phenol.
Phenoxides are enolates stabilised by aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
. Under normal circumstances, phenoxide is more reactive at the oxygen position, but the oxygen position is a "hard" nucleophile whereas the alpha-carbon positions tend to be "soft".
Reactions
 Phenol is highly reactive toward electrophilic aromatic substitution. The enhance nucleophilicity is attributed to donation pi electron density from O into the ring. Many groups can be attached to the ring, via halogenation, acylation, sulfonation, and related processes. Phenol's ring is so strongly activated that bromination and chlorination lead readily to polysubstitution. Phenol reacts with dilute nitric acid at room temperature to give a mixture of 2-nitrophenol and 4-nitrophenol while with concentrated nitric acid, additional nitro groups are introduced, e.g. to give
Phenol is highly reactive toward electrophilic aromatic substitution. The enhance nucleophilicity is attributed to donation pi electron density from O into the ring. Many groups can be attached to the ring, via halogenation, acylation, sulfonation, and related processes. Phenol's ring is so strongly activated that bromination and chlorination lead readily to polysubstitution. Phenol reacts with dilute nitric acid at room temperature to give a mixture of 2-nitrophenol and 4-nitrophenol while with concentrated nitric acid, additional nitro groups are introduced, e.g. to give 2,4,6-trinitrophenol
Picric acid is an organic compound with the formula (O2N)3C6H2OH. Its IUPAC name is 2,4,6-trinitrophenol (TNP). The name "picric" comes from el, πικρός (''pikros''), meaning "bitter", due to its bitter taste. It is one of the most acidic ...
.
Aqueous solutions of phenol are weakly acidic and turn blue litmus slightly to red. Phenol is neutralized by sodium hydroxide forming sodium phenate or phenolate, but being weaker than carbonic acid, it cannot be neutralized by sodium bicarbonate or sodium carbonate to liberate carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
.
:zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
dust or when its vapour is passed over granules of zinc at 400 °C:
:Production
Because of phenol's commercial importance, many methods have been developed for its production, but the cumene process is the dominant technology.Cumene process
 Accounting for 95% of production (2003) is the
Accounting for 95% of production (2003) is the cumene process
The cumene process (cumene-phenol process, Hock process) is an industrial process for synthesizing phenol and acetone from benzene and propylene. The term stems from cumene (isopropyl benzene), the intermediate material during the process. It w ...
, also called Hock process. It involves the partial oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
of cumene (isopropylbenzene) via the Hock rearrangement
Hock may refer to:
Common meanings:
* Hock (wine), a type of wine
* Hock (anatomy), part of an animal's leg
* To leave an item with a pawnbroker
People:
* Hock (surname)
* Richard "Hock" Walsh (1948-1999), Canadian blues singer
Other uses:
* A ...
: Compared to most other processes, the cumene process uses relatively mild conditions and relatively inexpensive raw materials. For the process to be economical, both phenol and the acetone by-product must be in demand. In 2010, worldwide demand for acetone was approximately 6.7 million tonnes, 83 percent of which was satisfied with acetone produced by the cumene process.
A route analogous to the cumene process begins with cyclohexylbenzene
Cyclohexylbenzene is the organic compound with the structural formula C6H5-C6H11. It is a derivative of benzene with a cyclohexyl substituent (C6H11). It is a colorless liquid.
Formation
Cyclohexylbenzene is produced by the acid-catalyzed alkyl ...
. It is oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
to a hydroperoxide
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. ...
, akin to the production of cumene hydroperoxide. Via the Hock rearrangement, cyclohexylbenzene hydroperoxide cleaves to give phenol and cyclohexanone. Cyclohexanone is an important precursor to some nylon
Nylon is a generic designation for a family of synthetic polymers composed of polyamides ( repeating units linked by amide links).The polyamides may be aliphatic or semi-aromatic.
Nylon is a silk-like thermoplastic, generally made from pet ...
s.
Oxidation of benzene and toluene
The direct oxidation of benzene () to phenol is theoretically possible and of great interest, but it has not been commercialized: :Nitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or nos, is a chemical compound, an oxide of nitrogen with the formula . At room temperature, it is a colourless non-flammable gas, and ha ...
is a potentially "green" oxidant that is a more potent oxidant than O2. Routes for the generation of nitrous oxide however remain uncompetitive.
An electrosynthesis employing alternating current gives phenol from benzene.
The oxidation of toluene, as developed by Dow Chemical, involves copper-catalyzed reaction of molten sodium benzoate with air:
:Older methods
Early methods relied on extraction of phenol from coal derivatives or the hydrolysis of benzene derivatives.Hydrolysis of benzenesulfonic acid
An early commercial route, developed by Bayer and Monsanto in the early 1900s, begins with the reaction of a strong base with benzenesulfonic acid. The conversion is represented by this idealized equation:Wittcoff, H.A., Reuben, B.G. Industrial Organic Chemicals in Perspective. Part One: Raw Materials and Manufacture. Wiley-Interscience, New York. 1980. :Hydrolysis of chlorobenzene
Chlorobenzene can be hydrolyzed to phenol using base ( Dow process) or steam ( Raschig–Hooker process): :Coal pyrolysis
Phenol is also a recoverable byproduct of coal pyrolysis.Franck, H.-G., Stadelhofer, J.W. Industrial Aromatic Chemistry. Springer-Verlag, New York. 1988. pp. 148-155. In theLummus Process Lummus may refer to:
* J. E. Lummus and J. N. Lummus, bankers who moved to Miami in 1895
*Lummus Company, manufacturer of cotton gins, Savannah Georgia
* Lummus Global, a company of Chicago Bridge & Iron Company
* Lummus Island, one of the three is ...
, the oxidation of toluene to benzoic acid is conducted separately.
Miscellaneous methods

Phenyldiazonium
Benzenediazonium tetrafluoroborate is an organic compound with the formula 6H5N2F4. It is a salt of a diazonium cation and tetrafluoroborate. It exists as a colourless solid that is soluble in polar solvents. It is the parent member of the aryl ...
salts hydrolyze to phenol. The method is of no commercial interest since the precursor is expensive.
:Uses
The major uses of phenol, consuming two thirds of its production, involve its conversion to precursors for plastics. Condensation with acetone givesbisphenol-A
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial s ...
, a key precursor to polycarbonates and epoxide resins. Condensation of phenol, alkylphenols, or diphenols with formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
gives phenolic resins, a famous example of which is Bakelite. Partial hydrogenation of phenol gives cyclohexanone, a precursor to nylon
Nylon is a generic designation for a family of synthetic polymers composed of polyamides ( repeating units linked by amide links).The polyamides may be aliphatic or semi-aromatic.
Nylon is a silk-like thermoplastic, generally made from pet ...
. Nonionic detergents are produced by alkylation of phenol to give the alkylphenols, e.g., nonylphenol, which are then subjected to ethoxylation.
Phenol is also a versatile precursor to a large collection of drugs, most notably aspirin but also many herbicides and pharmaceutical drugs.
Phenol is a component in liquid–liquid phenol–chloroform extraction technique used in molecular biology
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and phys ...
for obtaining nucleic acids from tissues or cell culture samples. Depending on the pH of the solution either DNA or RNA can be extracted.
Medical
Phenol is widely used as an antiseptic. Its use was pioneered by Joseph Lister (see section). From the early 1900s to the 1970s it was used in the production ofcarbolic soap
Carbolic soap, sometimes referred to as red soap, is a mildly antiseptic soap containing carbolic acid (phenol) and/or cresylic acid (cresol), both of which are phenols derived from either coal tar or petroleum sources.
History
In 1834, German ...
. Concentrated phenol liquids are commonly used for permanent treatment of ingrown toe and finger nails, a procedure known as a chemical matrixectomy. The procedure was first described by Otto Boll in 1945. Since that time it has become the chemical of choice for chemical matrixectomies performed by podiatrists.
Concentrated liquid phenol can be used topically as a local anesthetic for otology procedures, such as myringotomy and tympanotomy tube placement, as an alternative to general anesthesia or other local anesthetics. It also has hemostatic and antiseptic qualities that make it ideal for this use.
Phenol spray, usually at 1.4% phenol as an active ingredient, is used medically to treat sore throat. It is the active ingredient in some oral analgesics such as Chloraseptic
Chloraseptic is an American brand of oral analgesic that is produced by Tarrytown, New York-based Prestige Consumer Healthcare, used for the relief of sore throat and mouth pain. Its active ingredient is phenol (just in Sore Throat Spray, not i ...
spray, TCP and Carmex
Carmex is a brand of lip balm produced by Carma Laboratories, Inc. It is sold in jars, sticks, and squeezable containers.
History
Carma Laboratories, Inc. began in Wisconsin in the early 1930s when Alfred Woelbing began experimenting with creat ...
.
Niche uses
Phenol is so inexpensive that it attracts many small-scale uses. It is a component of industrial paint strippers used in the aviation industry for the removal of epoxy, polyurethane and other chemically resistant coatings. Phenol derivatives have been used in the preparation ofcosmetics
Cosmetics are constituted mixtures of chemical compounds derived from either natural sources, or synthetically created ones. Cosmetics have various purposes. Those designed for personal care and skin care can be used to cleanse or protec ...
including sunscreens, hair colorings, and skin lightening preparations. However, due to safety concerns, phenol is banned from use in cosmetic products in the European Union
The European Union (EU) is a supranational union, supranational political union, political and economic union of Member state of the European Union, member states that are located primarily in Europe, Europe. The union has a total area of ...
and Canada
Canada is a country in North America. Its ten provinces and three territories extend from the Atlantic Ocean to the Pacific Ocean and northward into the Arctic Ocean, covering over , making it the world's second-largest country by to ...
.
History
Phenol was discovered in 1834 by Friedlieb Ferdinand Runge, who extracted it (in impure form) fromcoal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat pso ...
. Runge called phenol "Karbolsäure" (coal-oil-acid, carbolic acid). Coal tar remained the primary source until the development of the petrochemical industry. The French chemist Auguste Laurent extracted phenol in its pure form, as a derivative of benzene, in 1841.
In 1836, Auguste Laurent coined the name "phène" for benzene; this is the root of the word "phenol" and " phenyl". In 1843, French chemist Charles Gerhardt coined the name "phénol".
The antiseptic properties of phenol were used by Sir Joseph Lister
Joseph Lister, 1st Baron Lister, (5 April 182710 February 1912) was a British surgeon, medical scientist, experimental pathologist and a pioneer of antiseptic surgery and preventative medicine. Joseph Lister revolutionised the craft of ...
(1827–1912) in his pioneering technique of antiseptic surgery. Lister decided that the wounds themselves had to be thoroughly cleaned. He then covered the wounds with a piece of rag or lint covered in carbolic acid (phenol). The skin irritation caused by continual exposure to phenol eventually led to the introduction of aseptic (germ-free) techniques in surgery.
Joseph Lister was a student at University College London under Robert Liston, later rising to the rank of Surgeon at Glasgow Royal Infirmary. Lister experimented with cloths covered in carbolic acid after studying the works and experiments of his contemporary, Louis Pasteur in sterilizing various biological media. Lister was inspired to try to find a way to sterilize living wounds, which could not be done with the heat required by Pasteur's experiments. In examining Pasteur's research, Lister began to piece together his theory: that patients were being killed by germs. He theorized that if germs could be killed or prevented, no infection would occur. Lister reasoned that a chemical could be used to destroy the micro-organisms that cause infection.
Meanwhile, in Carlisle, England, officials were experimenting with sewage treatment using carbolic acid to reduce the smell of sewage cesspools. Having heard of these developments, and having himself previously experimented with other chemicals for antiseptic purposes without much success, Lister decided to try carbolic acid as a wound antiseptic. He had his first chance on August 12, 1865, when he received a patient: an eleven-year-old boy with a tibia bone fracture which pierced the skin of his lower leg. Ordinarily, amputation would be the only solution. However, Lister decided to try carbolic acid. After setting the bone and supporting the leg with splints, he soaked clean cotton towels in undiluted carbolic acid and applied them to the wound, covered with a layer of tin foil, leaving them for four days. When he checked the wound, Lister was pleasantly surprised to find no signs of infection, just redness near the edges of the wound from mild burning by the carbolic acid. Reapplying fresh bandages with diluted carbolic acid, the boy was able to walk home after about six weeks of treatment.
By 16 March 1867, when the first results of Lister's work were published in the Lancet, he had treated a total of eleven patients using his new antiseptic method. Of those, only one had died, and that was through a complication that was nothing to do with Lister's wound-dressing technique. Now, for the first time, patients with compound fractures were likely to leave the hospital with all their limbs intact :— Richard Hollingham, ''Blood and Guts: A History of Surgery'', p. 62
Before antiseptic operations were introduced at the hospital, there were sixteen deaths in thirty-five surgical cases. Almost one in every two patients died. After antiseptic surgery was introduced in the summer of 1865, there were only six deaths in forty cases. The mortality rate had dropped from almost 50 per cent to around 15 per cent. It was a remarkable achievement :— Richard Hollingham, ''Blood and Guts: A History of Surgery'', p. 63Phenol was the main ingredient of the Carbolic Smoke Ball, an ineffective device marketed in London in the 19th century as protection against influenza and other ailments, and the subject of the famous law case '' Carlill v Carbolic Smoke Ball Company''.
Second World War
The toxic effect of phenol on the central nervous system, discussed below, causes sudden collapse and loss of consciousness in both humans and animals; a state of cramping precedes these symptoms because of the motor activity controlled by the central nervous system. Injections of phenol were used as a means of individual execution byNazi Germany
Nazi Germany (lit. "National Socialist State"), ' (lit. "Nazi State") for short; also ' (lit. "National Socialist Germany") (officially known as the German Reich from 1933 until 1943, and the Greater German Reich from 1943 to 1945) was ...
during the Second World War
World War II or the Second World War, often abbreviated as WWII or WW2, was a world war that lasted from 1939 to 1945. It involved the vast majority of the world's countries—including all of the great powers—forming two opposi ...
.''The Experiments''by Peter Tyson. NOVA It was originally used by the Nazis in 1939 as part of the Aktion T4 euthanasia program.''The Nazi Doctors''
, Chapter 14, Killing with Syringes: Phenol Injections. By Dr. Robert Jay Lifton The Germans learned that extermination of smaller groups was more economical by injection of each victim with phenol. Phenol injections were given to thousands of people.
Maximilian Kolbe
Maximilian Maria Kolbe (born Raymund Kolbe; pl, Maksymilian Maria Kolbe; 1894–1941) was a Polish Catholic priest and Conventual Franciscan friar who volunteered to die in place of a man named Franciszek Gajowniczek in the German death cam ...
was also killed with a phenol injection after surviving two weeks of dehydration and starvation in Auschwitz when he volunteered to die in place of a stranger
''A Stranger'' ( hr, Obrana i zaštita; ) is a 2013 Croatian drama film directed by Bobo Jelčić.
Cast
* Bogdan Diklić — Slavko
* Nada Đurevska — Milena
* Ivana Roščić — Zehra
* Rakan Rushaidat — Krešo
* Vinko Kraljević — Mi ...
. Approximately one gram is sufficient to cause death.
Occurrences
Phenol is a normal metabolic product, excreted in quantities up to 40 mg/L in human urine. The temporal gland secretion of maleelephant
Elephants are the largest existing land animals. Three living species are currently recognised: the African bush elephant, the African forest elephant, and the Asian elephant. They are the only surviving members of the family Elephantida ...
s showed the presence of phenol and 4-methylphenol during musth.
It is also one of the chemical compounds found in castoreum
Castoreum is a yellowish exudate from the castor sacs of mature beavers. Beavers use castoreum in combination with urine to scent mark their territory. Both beaver sexes have a pair of castor sacs and a pair of anal glands, located in two cavities ...
. This compound is ingested from the plants the beaver eats.
Occurrence in whisky
Phenol is a measurable component in the aroma and taste of the distinctive Islay scotch whisky, generally ~30 ppm, but it can be over 160ppm in the malted barley used to produce whisky. This amount is different from and presumably higher than the amount in the distillate.Biodegradation
'' Cryptanaerobacter phenolicus'' is a bacterium species that producesbenzoate
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, wh ...
from phenol via 4-hydroxybenzoate
4-Hydroxybenzoic acid, also known as ''p''-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar ...
. '' Rhodococcus phenolicus'' is a bacterium species able to degrade phenol as sole carbon source.
Toxicity
Phenol and its vapors are corrosive to the eyes, the skin, and the respiratory tract. Its corrosive effect on skin and mucous membranes is due to a protein-degenerating effect. Repeated or prolonged skin contact with phenol may causedermatitis
Dermatitis is inflammation of the skin, typically characterized by itchiness, redness and a rash. In cases of short duration, there may be small blisters, while in long-term cases the skin may become thickened. The area of skin involved c ...
, or even second and third-degree burns. Inhalation of phenol vapor may cause lung edema. The substance may cause harmful effects on the central nervous system and heart, resulting in dysrhythmia, seizures, and coma. The kidney
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blo ...
s may be affected as well. Long-term or repeated exposure of the substance may have harmful effects on the liver
The liver is a major organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it i ...
and kidney
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blo ...
s. There is no evidence that phenol causes cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
in humans. Besides its hydrophobic effects, another mechanism for the toxicity of phenol may be the formation of phenoxyl radicals
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
.
Since phenol is absorbed through the skin relatively quickly, systemic poisoning can occur in addition to the local caustic burns. Resorptive poisoning by a large quantity of phenol can occur even with only a small area of skin, rapidly leading to paralysis of the central nervous system and a severe drop in body temperature. The for oral toxicity is less than 500 mg/kg for dogs, rabbits, or mice; the minimum lethal human dose was cited as 140 mg/kg. The Agency for Toxic Substances and Disease Registry (ATSDR), U.S. Department of Health and Human Services states the fatal dose for ingestion of phenol is from 1 to 32 g.
Chemical burns from skin exposures can be decontaminated by washing with polyethylene glycol, isopropyl alcohol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group ( chemical formula ) it is the s ...
, or perhaps even copious amounts of water. Removal of contaminated clothing is required, as well as immediate hospital treatment for large splashes. This is particularly important if the phenol is mixed with chloroform (a commonly used mixture in molecular biology for DNA and RNA purification). Phenol is also a reproductive toxin causing increased risk of miscarriage and low birth weight indicating retarded development in utero.
Phenols
The word ''phenol'' is also used to refer to any compound that contains a six-memberedaromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
ring, bonded directly to a hydroxyl group (-OH). Thus, phenols are a class of organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
s of which the phenol discussed in this article is the simplest member.
See also
* Bamberger rearrangement * Claisen rearrangement * Cresol *Fries rearrangement
The Fries rearrangement, named for the German chemist Karl Theophil Fries, is a rearrangement reaction of a phenolic ester to a hydroxy aryl ketone by catalysis of Lewis acids.
It involves migration of an acyl group of phenol ester to the aryl ...
* Polyphenol
References
External links
International Chemical Safety Card 0070
{{Authority control Antiseptics Commodity chemicals Hazardous air pollutants Oxoacids Phenyl compounds 1834 in science