organophosphine on:
[Wikipedia]
[Google]
[Amazon]
Organophosphines are
organophosphorus compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective in ...
s with the formula PR''n''H3−''n'', where R is an organic substituent. These compounds can be classified according to the value of ''n'': primary phosphines (''n'' = 1), secondary phosphines (''n'' = 2), tertiary phosphines (''n'' = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
(PH3).
Annette Schier and Hubert Schmidbaur"P-Donor Ligands" in Encyclopedia of Inorganic Chemistry 2006, Wiley-VCH, Weinheim.
1° vs 2° vs 3° phosphines
Organophophines are classified according to the number of organic substituents.Primary phosphines
Primary (1°) phosphines, with the formula RPH2, are typically prepared by alkylation of phosphine. Simple alkyl derivatives such as methylphosphine (CH3PH2) are prepared by alkylation of alkali metal derivatives MPH2 (M is Li, Na, or K). Another synthetic route involves treatment of the corresponding chlorophosphines with hydride reagents. For example, reduction ofdichlorophenylphosphine
Dichlorophenylphosphine is an organophosphorus compound with the formula C6H5PCl2. This colourless viscous liquid is commonly used in the synthesis of organophosphines.
Dichlorophenylphosphine is commercially available. It may be prepared by an ...
with lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li Al H4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic ...
affords phenylphosphine (PhPH2).
Primary (RPH2) and secondary phosphines (RRPH and R2PH) add to alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s in presence of a strong base (e.g., KOH in DMSO
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds an ...
). Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Explanation
The rule states that with the addition of a ...
s apply. Similar reactions occur involving alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s. Base is not required for electron-deficient alkenes (e.g., derivatives of acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecular st ...
) and alkynes.
:
Secondary phosphines
Secondary (2°) phosphines, with the formula R2PH, are prepared analogously to the primary phosphines. They are also obtained by alkali-metal reductive cleavage of triarylphosphines followed by hydrolysis of the resulting phosphide salt. The latter route is employed to prepare diphenylphosphine (Ph2PH). Diorganophosphinic acids, R2P(O)OH, can also be reduced withdiisobutylaluminium hydride
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH) is a reducing agent with the formula (''i''-Bu2AlH)2, where ''i''-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound is a reagent in organic synthesis.
Properties
Lik ...
. Secondary phosphines are typically protic in character.
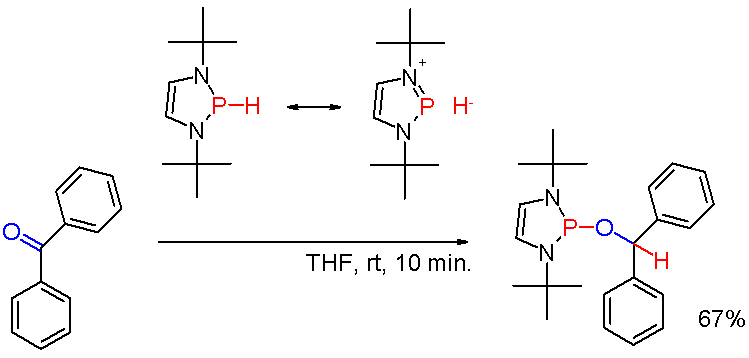
But when modified with suitable substituents, as in certain (rare) ''diazaphospholenes'' (''scheme 3''), the polarity of the P-H bond can be inverted (see: umpolung
In organic chemistry, umpolung () or polarity inversion is the chemical modification of a functional group with the aim of the reversal of polarity of that group. This modification allows secondary reactions of this functional group that would ot ...
) and the resulting phosphine hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
can reduce a carbonyl group as in the example of benzophenone
Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. It is a white solid that is soluble in organic solvents. Benzophenone is a widely used building block in organic chemistry, being the parent diarylket ...
in yet another way.
Secondary phosphines occur in cyclic forms. Three-membered rings are phosphirane
Phosphirane is the organophosphorus compound with the formula C2H4PH. It is a colorless gas of no commercial value. As the simplest cyclic, saturated organophosphorus compound, phosphirane is the prototype of a family of related compounds that ha ...
s (unsaturated: phosphirene
Phosphirene is the hypothetical organophosphorus compound with the formula C2H2PH. As the simplest cyclic, unsaturated organophosphorus compound, phosphirene is the prototype of a family of related compounds that have attracted attention from rese ...
s), five-membered rings are phospholanes (unsaturated: phosphole
Phosphole is the organic compound with the chemical formula ; it is the phosphorus analog of pyrrole. The term phosphole also refers to substituted derivatives of the parent heterocycle. These compounds are of theoretical interest but also serve a ...
), and six-membered rings are phosphinanes.

Tertiary phosphines
Tertiary (3°) phosphines, with the formula R3P, are traditionally prepared by alkylation ofphosphorus trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic ...
using Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
s or related organolithium
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
compounds:
:3RMgX + PCl3 → PR3 + 3MgX2
In the case of trimethylphosphine
Trimethylphosphine is a neutral organophosphorus compound with the formula P(CH3)3, commonly abbreviated as PMe3. This colorless liquid has a strongly unpleasant odor, characteristic of alkylphosphines. The compound is a common ligand in coordin ...
, triphenyl phosphite is used in place of the highly electrophilic PCl3:
: 3 CH3MgBr + P(OC6H5)3 → P(CH3)3 + 3 C6H5OMgBr
Slightly more elaborate methods are employed for the preparation of unsymmetrical tertiary phosphines, with the formula R2R'P. The use of organophosphorus-based nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
s is typical. For example, lithium diphenylphosphide
Lithium diphenylphosphide contains lithium and the organophosphorus anion with the formula (C6H5)2PLi. It is an air-sensitive solid that is used in the preparation of diphenylphosphino compounds. As an ether complex, the lithium salt is dark red ...
is readily methylated with methyl iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one ...
to give methyldiphenylphosphine:
:LiiP(C6H5)2 + CH3I → CH3P(C6H5)2 + LiI
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
is a precursor to some tertiary phosphines by hydrophosphination of alkenes. For example, in the presence of basic catalysts PH3 adds of Michael acceptor
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon ...
s such as acrylonitrile
Acrylonitrile is an organic compound with the formula and the structure . It is a colorless, volatile liquid although commercial samples can be yellow due to impurities. It has a pungent odor of garlic or onions. In terms of its molecular st ...
:
:PH3 + 3 CH2=CHZ → P(CH2CH2Z)3 (Z = NO2, CN, C(O)NH2)
Tertiary phosphines of the type PRR′R″ are " ''P''-chiral" and optically stable.
From the commercial perspective, the most important phosphine is triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists a ...
, several million kilograms being produced annually. It is prepared from the reaction of chlorobenzene
Chlorobenzene is an aromatic organic compound with the chemical formula C6H5Cl. This colorless, flammable liquid is a common solvent and a widely used intermediate in the manufacture of other chemicals.
Uses
Historical
The major use of chlorob ...
, PCl3, and sodium. Phosphines of a more specialized nature are usually prepared by other routes.
Di- and triphosphiines

Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backb ...
are also available in primary, secondary, and tertiary phosphorus substituents. Triphosphines etc. are similar.
Structure and bonding
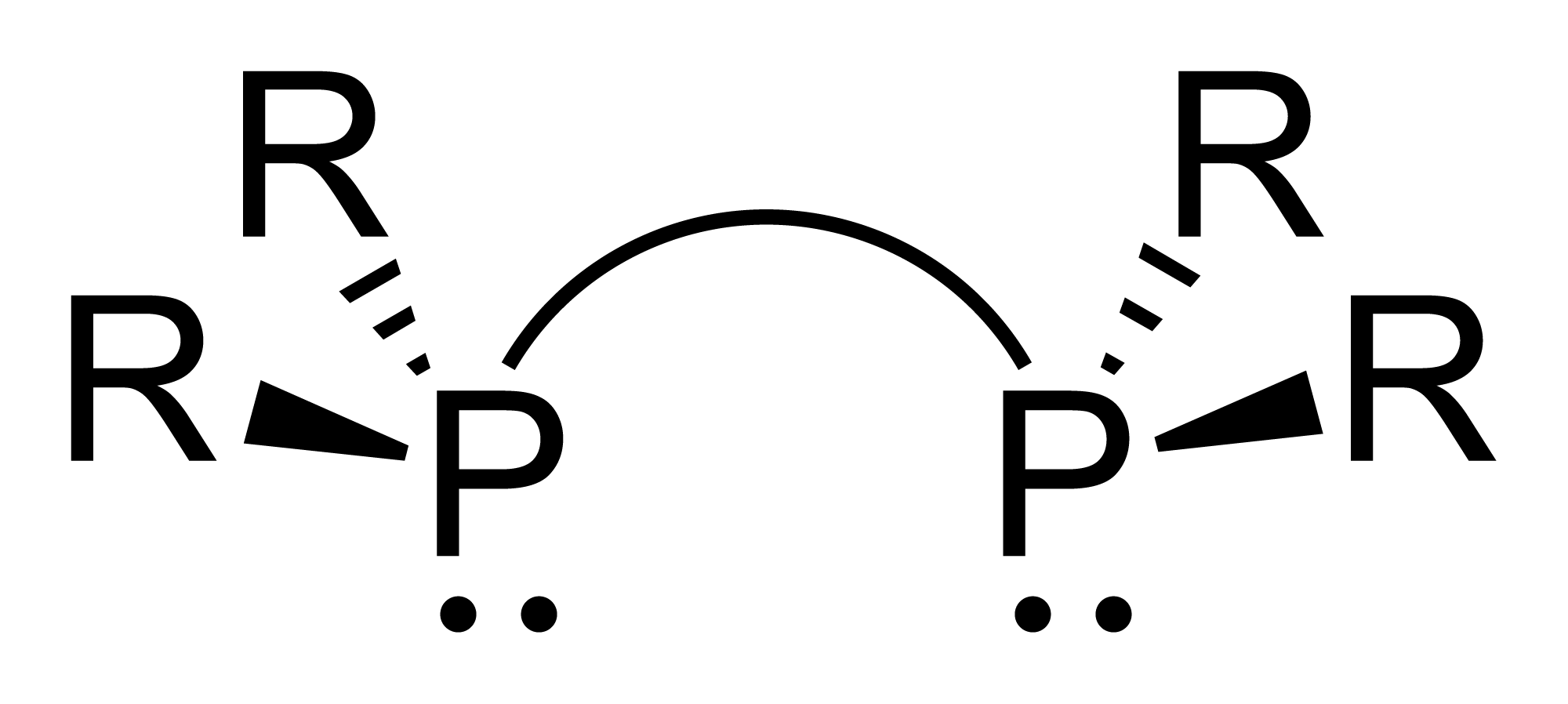
Organophosphines, like phosphine itself, are pyramidal molecules with approximate ''C''3''v''symmetry
Symmetry (from grc, συμμετρία "agreement in dimensions, due proportion, arrangement") in everyday language refers to a sense of harmonious and beautiful proportion and balance. In mathematics, "symmetry" has a more precise definiti ...
. The C–P–C bond angles are approximately 98.6°. The C–P–C bond angles are consistent with the notion that phosphorus predominantly uses the 3p orbitals for forming bonds and that there is little sp hybridization of the phosphorus atom. The latter is a common feature of the chemistry of phosphorus. As a result, the lone pair of trimethylphosphine has predominantly s-character as is the case for phosphine, PH3.
Tertiary phosphines are pyramidal. When the organic substituents all differ, the phosphine is chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
and configurationally stable (in contrast to NRR'R"). Complexes derived from the chiral phosphines can catalyse reactions to give chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
, enantioenriched products.
Comparison of phosphines and amines
The phosphorus atom in phosphines has a formal oxidation state −3 (σ3λ3) and are the phosphorus analogues ofamine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such ...
s. Like amines, phosphines have a trigonal pyramidal molecular geometry
In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base, resembling a tetrahedron (not to be confused with the tetrahedral geometry). When all three atoms at the cor ...
although often with smaller C-E-C angles (E = N, P), at least in the absence of steric effects. The C-P-C bond angle
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Chemical bond, the attraction of atoms, ions or molecules to form chemical ...
is 98.6° for trimethylphosphine increasing to 109.7° when the methyl groups are replaced by ''tert''-butyl groups. When used as ligands, the steric bulk of tertiary phosphines is evaluated by their cone angle
In coordination chemistry, the ligand cone angle (a common example being the Tolman cone angle or ''θ'') is a measure of the steric bulk of a ligand in a transition metal coordination complex. It is defined as the solid angle formed with the m ...
. The barrier to pyramidal inversion is also much higher than nitrogen inversion In chemistry, pyramidal inversion (also umbrella inversion) is a fluxional process in compounds with a pyramidal molecule, such as ammonia (NH3) "turns inside out". It is a rapid oscillation of the atom and substituents, the molecule or ion passi ...
to occur, and therefore phosphines with three different substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as '' side ...
s can be resolved into thermally stable optical isomer
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (). The terms ar ...
s. Phosphines are often less basic than corresponding amines, for instance the phosphonium ion itself has a p''K''a of −14 compared to 9.21 for the ammonium ion; trimethylphosphonium has a p''K''a of 8.65 compared to 9.76 for trimethylammonium. However, triphenylphosphine (p''K''a 2.73) is more basic than triphenylamine (p''K''a −5), mainly because the lone pair of the nitrogen in NPh3 is partially delocalized into the three phenyl rings. Whereas the lone pair on nitrogen is delocalized in pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methy ...
, the lone pair on phosphorus atom in the phosphorus equivalent of pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methy ...
(phosphole
Phosphole is the organic compound with the chemical formula ; it is the phosphorus analog of pyrrole. The term phosphole also refers to substituted derivatives of the parent heterocycle. These compounds are of theoretical interest but also serve a ...
) is not. The reactivity of phosphines matches that of amines with regard to nucleophilicity in the formation of phosphonium salts with the general structure PR4+X−. This property is used in the Appel reaction
The Appel reaction is an organic reaction that converts an alcohol into an alkyl chloride using triphenylphosphine and carbon tetrachloride. The use of carbon tetrabromide or bromine as a halide source will yield alkyl bromides, whereas using car ...
for converting alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of se ...
s to alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
s. Phosphines are easily oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
to the corresponding phosphine oxides, whereas amine oxides are less readily generated. In part for this reason, phosphines are very rarely encountered in nature.
Reactions
Coordination chemistry
Tertiary phosphines are often used asligands
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elec ...
in coordination chemistry. The binding of phosphines bind to metals, which serve as Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s. For example, silver chloride
Silver chloride is a chemical compound with the chemical formula Ag Cl. This white crystalline solid is well known for its low solubility in water (this behavior being reminiscent of the chlorides of Tl+ and Pb2+). Upon illumination or heating, ...
reacts with triphenylphosphine to 1;1 and 1:2 complexes:
:PPh3 + AgCl → ClAgPPh3
:PPh3 + ClAgPPh3 → ClAg(PPh3)2
The adducts formed from phosphines and borane are useful reagents. These phosphine-boranes are air-stable, but the borane protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In ma ...
can be removed by treatment with amines.
Quaternization
Akin to complexation, phosphines are readily alkylated. For example,methyl bromide
Bromomethane, commonly known as methyl bromide, is an organobromine compound with formula C H3 Br. This colorless, odorless, nonflammable gas is produced both industrially and biologically. It has a tetrahedral shape and it is a recognized ozo ...
converts triphenylphosphine to the methyltriphenylphosphonium bromide, a "quat salt":
:PPh3 + CH3Br → H3PPh3+r−
Phosphines are nucleophilic catalysts in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
, e.g. the Rauhut–Currier reaction and Baylis-Hillman reaction.
Protonation and deprotonation
Like phosphine itself, but easier, organophosphines undergo protonation. The reaction is reversible. Whereas organophosphines are oxygen-sensitive, the protonated derivatives are not. Primary and secondary derivatives, they can be deprotonated by strong bases to give organophosphide
In chemistry, a phosphide is a compound containing the ion or its equivalent. Many different phosphides are known, with widely differing structures. Most commonly encountered on the binary phosphides, i.e. those materials consisting only of phos ...
derivatives. Thus diphenylphosphine reacts with organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
to give lithium diphenylphosphide
Lithium diphenylphosphide contains lithium and the organophosphorus anion with the formula (C6H5)2PLi. It is an air-sensitive solid that is used in the preparation of diphenylphosphino compounds. As an ether complex, the lithium salt is dark red ...
:
:HPPh2 + RLi → LiPPh2 + RH
Oxidation and sulfiding
Tertiary phosphines characteristically oxidize to give phosphine oxides with the formula R3PO. The reaction with oxygen is spin-forbidden but still proceeds at sufficient rate that samples of tertiary phosphines are characteristically contaminated with phosphine oxides. Qualitatively, the rates of oxidation are higher for trialkyl vs triarylphosphines. Faster still are oxidations usinghydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3 ...
. Primary and secondary phosphines also oxidize, but the product(s) are subject to tautomerization and further oxidation.
Tertiary phosphines characteristically oxidize to give phosphine sulfides.
The reducing properties of organophosphiines is also illustrated in the Staudinger reduction for the conversion of organic azides to amines and in the Mitsunobu reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylat ...
for converting alcohols into esters. In these processes, the phosphine is oxidized to phosphorus(V). Phosphines have also been found to reduce activated carbonyl groups, for instance the reduction of an α-keto ester to an α-hydroxy ester in ''scheme 2''. In the proposed reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
, the first proton is on loan from the methyl group in trimethylphosphine (triphenylphosphine does not react).
:
See also
*Diphosphines
Diphosphines, sometimes called bisphosphanes, are organophosphorus compounds most commonly used as bidentate phosphine ligands in inorganic and organometallic chemistry. They are identified by the presence of two phosphino groups linked by a backb ...
, R2PPR2, R2P(CH2)''n''PR2
* Phosphine oxide, R3P=O
*Phosphorane
A phosphorane (IUPAC name: λ5-phosphane) is a functional group in organophosphorus chemistry with pentavalent phosphorus. It has the general formula PR5. The parent hydride compound is the hypothetical molecule PH5. The derivative pentaphenylph ...
, PR5, R3P=CR2
*Phosphinite
In organic chemistry, phosphinites are organophosphorus compounds with the formula . They are used as ligands in homogeneous catalysis and coordination chemistry.
Preparation
Phosphinites are prepared by alcoholysis of organophosphinous chlori ...
, P(OR)R2
*Phosphonite
In organic chemistry, phosphonites are organophosphorus compounds with the formula P(OR)2R. They are found in some pesticides and are used as ligands.
Preparation
Although they are derivatives of phosphonous acid (RP(OH)2), they are not prepar ...
, P(OR)2R
*Phosphite
The general structure of a phosphite ester showing the lone pairs on the P
In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of ...
, P(OR)3
* Phosphinate, R2P(RO)O
*Phosphonate
In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing groups (where R = alkyl, aryl, or just hydrogen). Phosphonic acids, typically handled as salts, are generally nonvolatile solids that are poo ...
, RP(RO)2O
References
{{Reflist Functional groups Phosphorus(−III) compounds