organoboranes on:
[Wikipedia]
[Google]
[Amazon]
 Organoborane or organoboron compounds are
Organoborane or organoboron compounds are

 Hydroboration of alkenes or alkynes is an efficient method for the generation of boranes; however, the use of borane (BH3) or borane equivalents leads to the conversion of only 33% of the starting olefin to product after oxidation or protonolysis—the remaining olefin is incorporated into boron-containing byproducts. The use of a stoichiometric amount of 9-borabicyclo .3.1onane (9-BBN) as the hydroborating reagent provides a solution to this problem.
Hydroboration of alkenes or alkynes is an efficient method for the generation of boranes; however, the use of borane (BH3) or borane equivalents leads to the conversion of only 33% of the starting olefin to product after oxidation or protonolysis—the remaining olefin is incorporated into boron-containing byproducts. The use of a stoichiometric amount of 9-borabicyclo .3.1onane (9-BBN) as the hydroborating reagent provides a solution to this problem.

 α-Halo enolates are commonly used as nucleophiles in this context. After nucleophilic attack at boron, the resulting ketoboronate rearranges to a neutral enolborane. Upon protonolysis, a functionalized carbonyl compound results. The intermediate enolboranes may also be quenched with electrophiles.
α-Halo enolates are commonly used as nucleophiles in this context. After nucleophilic attack at boron, the resulting ketoboronate rearranges to a neutral enolborane. Upon protonolysis, a functionalized carbonyl compound results. The intermediate enolboranes may also be quenched with electrophiles.
 Alkynylboronates are versatile intermediates that can be converted to either ketones or olefins after simultaneous migration and attack of the alkyne on a separate electrophile. The electrophile and migrating group end up ''trans'' in the resulting alkenylborane. Protonolysis of this intermediate generates olefins, while oxidation leads to ketones after tautomerization.
Alkynylboronates are versatile intermediates that can be converted to either ketones or olefins after simultaneous migration and attack of the alkyne on a separate electrophile. The electrophile and migrating group end up ''trans'' in the resulting alkenylborane. Protonolysis of this intermediate generates olefins, while oxidation leads to ketones after tautomerization.

 Tertiary alcohols with two identical groups attached to the alcohol carbon may be synthesized through a double migration reaction of alkynylborates in the presence of acid.Midland, M. M.; Brown, H. C. ''J. Org. Chem.'' 1975, ''40'', 2845. Use of a single equivalent of acid and oxidation or protonolysis leads to ketones or olefins, respectively (see Mechanism and Stereochemistry section above).
Tertiary alcohols with two identical groups attached to the alcohol carbon may be synthesized through a double migration reaction of alkynylborates in the presence of acid.Midland, M. M.; Brown, H. C. ''J. Org. Chem.'' 1975, ''40'', 2845. Use of a single equivalent of acid and oxidation or protonolysis leads to ketones or olefins, respectively (see Mechanism and Stereochemistry section above).
 Acylation of borates is possible in the presence of an acyl halide. Here, the borate was generated from tri(cyclopentyl)borane and phenyllithium; the three cyclopentyl groups are serving as "dummy" groups and do not migrate to a significant amount.
Acylation of borates is possible in the presence of an acyl halide. Here, the borate was generated from tri(cyclopentyl)borane and phenyllithium; the three cyclopentyl groups are serving as "dummy" groups and do not migrate to a significant amount.
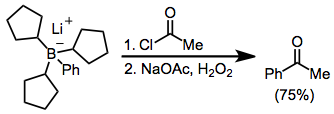 Treatment of trialkylboranes with α-halo enolates leads to functionalized ketones.Brown, H. C.; Rogi, M. M.; Nambu, H.; Rathke, M. W. ''J. Am. Chem. Soc.'' 1969, ''91'', 2147. Because the migration is stereospecific (retentive with respect to the migrating group and invertive at the α carbon), this method provides a means for the synthesis of enantiopure α-alkyl or -aryl ketones.
Treatment of trialkylboranes with α-halo enolates leads to functionalized ketones.Brown, H. C.; Rogi, M. M.; Nambu, H.; Rathke, M. W. ''J. Am. Chem. Soc.'' 1969, ''91'', 2147. Because the migration is stereospecific (retentive with respect to the migrating group and invertive at the α carbon), this method provides a means for the synthesis of enantiopure α-alkyl or -aryl ketones.
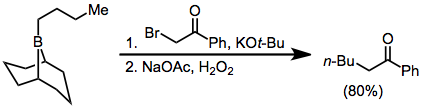 α-Halo ester enolates also add to boranes to eventually afford α-functionalized products; however, yields are slightly lower. Diazoesters and diazoketones may also be used in this context without the requirement for external base. α,α'-Dialo enolates react with boranes to form α-halo carbonyl compounds that can be further functionalized at the α position.
α-Halo ester enolates also add to boranes to eventually afford α-functionalized products; however, yields are slightly lower. Diazoesters and diazoketones may also be used in this context without the requirement for external base. α,α'-Dialo enolates react with boranes to form α-halo carbonyl compounds that can be further functionalized at the α position.
 Halides may be synthesized from organoboranes by activating with hydroxide or alkoxide and treatment with X2. Two of the three alkyl groups attached to the borane may be converted to halide in the presence of excess base, but the use of disiamylborane as the hydroborating reagent permits the selective halogenation of only the hydroborated olefin.
Halides may be synthesized from organoboranes by activating with hydroxide or alkoxide and treatment with X2. Two of the three alkyl groups attached to the borane may be converted to halide in the presence of excess base, but the use of disiamylborane as the hydroborating reagent permits the selective halogenation of only the hydroborated olefin.
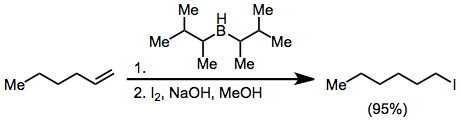 Treatment of an alkenylborane with iodine or bromine leads to migration of one of the organic groups attached to boron. Alkynyl groups migrate selectively, forming enynes after treatment with sodium acetate and hydrogen peroxide.Negishi, E.-i.; Lew, G.; Yoshida, T. ''Chem. Commun.'' 1973, 874.
Treatment of an alkenylborane with iodine or bromine leads to migration of one of the organic groups attached to boron. Alkynyl groups migrate selectively, forming enynes after treatment with sodium acetate and hydrogen peroxide.Negishi, E.-i.; Lew, G.; Yoshida, T. ''Chem. Commun.'' 1973, 874.

chemical compounds
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
of boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.
Organoboron compounds are important reagents in organic chemistry enabling many chemical transformations, the most important one called hydroboration. Reactions of organoborates and boranes involve the transfer of a nucleophilic group attached to boron to an electrophilic center either inter- or intramolecularly. α,β-Unsaturated borates, as well as borates with a leaving group at the α position, are highly susceptible to intramolecular 1,2-migration of a group from boron to the electrophilic α position. Oxidation or protonolysis
Protonolysis is the cleavage of a chemical bond by acids. Many examples are found in organometallic chemistry since the reaction requires polar Mδ+-Rδ- bonds, where δ+ and δ- signify partial positive and negative charges associated with the bon ...
of the resulting organoboranes may generate a variety of organic products, including alcohols, carbonyl compounds, alkenes, and halides.
Properties of the B-C bond
The C-B bond has low polarity (the difference inelectronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
2.55 for carbon and 2.04 for boron), and therefore alkyl boron compounds are in general stable though easily oxidized.
In part because its lower electronegativity, boron often forms electron-deficient compounds, such as the triorganoboranes. Vinyl group
In organic chemistry, a vinyl group (abbr. Vi; IUPAC name: ethenyl group) is a functional group with the formula . It is the ethylene (IUPAC name: ethene) molecule () with one fewer hydrogen atom. The name is also used for any compound contain ...
s and aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
groups donate electrons and make boron less electrophilic and the C-B bond gains some double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
character. Like the parent borane, diborane
Diborane(6), generally known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Diborane is a key boron compound with a variety of applications. It has attracte ...
, organoboranes are classified in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
as strong electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carri ...
s because boron is unable to gain a full octet
Octet may refer to:
Music
* Octet (music), ensemble consisting of eight instruments or voices, or composition written for such an ensemble
** String octet, a piece of music written for eight string instruments
*** Octet (Mendelssohn), 1825 com ...
of electrons. Unlike diborane however, most organoboranes do not form dimers.
Synthesis
From Grignard reagents
Simple organoboranes such astriethylborane
Triethylborane (TEB), also called triethylboron, is an organoborane (a compound with a B–C bond). It is a colorless pyrophoric liquid. Its chemical formula is or , abbreviated . It is soluble in organic solvents tetrahydrofuran and hexane.
P ...
or tris(pentafluorophenyl)boron can be prepared from trifluoroborane (as the ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again b ...
complex) and the ethyl or pentafluorophenyl Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . ...
. The borates (R4B−) are generated via addition of R−-equivalents (RMgX, RLi, etc.) to R3B.
From alkenes
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, a ...
s insert into B-H bonds of boranes in a process called hydroboration. The process involves anti- Markovnikov addition. Hydroboration of alkenes or alkynes with borane (BH3) or borane equivalents leads to the conversion of only 33% of the starting olefin to product after oxidation or protonolysis—the remaining olefin is incorporated into boron-containing byproducts. One organoboron reagent that is often employed in synthesis is 9-BBN. Hydroborations take place stereospecifically in a ''syn'' mode, that is on the same face of the alkene. In this concerted reaction
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend ...
the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
is represented as a square with the corners occupied by carbon, carbon, hydrogen and boron with maximum overlap between the two olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, a ...
p-orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
s and the empty boron orbital.
By borylation
Metal-catalyzed C-H Borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through functionalization of aliphatic and aromatic C-H bonds. A common reagent in this type of reaction is bis(pinacolato)diboron.Classes of organoboron compounds
Organoboranes and hydrides
Among the most studied classes of organoboron compounds have the formula BRnH3−n. As discussed above, these compounds are used as catalysts, reagents, and synthetic intermediates. The trialkyl and triaryl derivatives feature trigonal planar boron center that is typically only weaklyLewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
ic. Except for a few very bulky derivatives, the hydrides (BRnH3−n for n = 1 or 2) exist as dimers, reminiscent of the structure of diborane itself. Trisubstituted derivatives, e.g. triethylboron are monomers.
Borinic and boronic acids and esters (BRn(OR)3-n)
Compounds of the type BRn(OR)3-n are called borinic esters (n = 2),boronic ester
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, memb ...
s (n = 1), and borate
A borate is any of several boron oxyanions, negative ions consisting of boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt with such anions, such as sodium metaborate, and disodium tetraborate . The name also ...
s (n = 0). Boronic acids are used in Suzuki reaction
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, a ...
. Trimethyl borate
Trimethyl borate is the organoboron compound with the formula B(OCH3)3. It is a colourless liquid that burns with a green flame. It is an intermediate in the preparation of sodium borohydride and is a popular reagent in organic chemistry. It is a ...
, which is debatably not an organoboron compound, is an intermediate in the production of sodium borohydride.
Boron clusters
Boron is renowned for forming cluster compounds, e.g. dodecaborate 12H12sup>2-. Many organic derivatives are known for such clusters. One example is 12(CH3)12sup>2- and its radical derivative 12(CH3)12sup>−. Related cluster compounds with carbon vertices are calledcarborane
Carboranes are electron-delocalized (non-classically bonded) clusters composed of boron, carbon and hydrogen atoms.Grimes, R. N., ''Carboranes 3rd Ed.'', Elsevier, Amsterdam and New York (2016), . Like many of the related boron hydrides, these ...
s. The best known is orthocarborane, with the formula C2B10H12. Although they have few commercial applications, carboranes have attracted much attention because they are so structurally unusual. Anionic derivatives, dicarbollides, e.g., 2B9H11sup>2− are ligands that behave like cyclopentadienide
In chemistry, the cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of and abbreviated as Cp−. It is formed from the deprotonation of the molecule cyclopentadiene.
Properties
The cyclopentadienyl anion i ...
.
Bora-substituted aromatic compounds
Inborabenzene
Borabenzene is a hypothetical organoboron compound with the formula C5H5B. Unlike the related but highly stable benzene molecule, borabenzene would be electron-deficient. Related derivatives are the boratabenzene anions, including the parent 5H ...
, one CH center in benzene is replaced by boron. These compounds are invariably isolated as adducts, e.g., C5H5B-pyridine. The cyclic compound borole
Boroles represent a class of molecules known as metalloles, which are heterocyclic 5-membered rings. As such, they can be viewed as structural analogs of cyclopentadiene, pyrrole or furan, with boron replacing a carbon, nitrogen and oxygen atom re ...
, a structural analog
A structural analog (analogue in modern traditional English; Commonwealth English), also known as a chemical analog or simply an analog, is a compound having a structure similar to that of another compound, but differing from it in respect to a c ...
of pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methy ...
, has not been isolated, but substituted derivatives known as boroles are known. The cyclic compound borepin
Borepins are a class of boron-containing heterocycles used in main group chemistry. They consist of a seven-membered unsaturated ring with a tricoordinate boron in it. Simple borepins are analogues of cycloheptatriene, which is a seven-membered ...
is aromatic.
Boryl compounds
Boryl anions have the formula R2B−. Nucleophilic anionic boryl compounds have long been elusive but a 2006 study described a boryllithium compound, which reacts as anucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
: Organometallic compounds
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
with metal to boron bonds, (i.e., M–BR2), are known as boryl complexes. Related ligands are borylenes (M–B(R)–M).
:
The absence of lithium boryl compounds is notable because in other period 2 element
A period 2 element is one of the chemical elements in the second row (or period) of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of t ...
s lithium salts are common e.g. lithium fluoride
Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid, that transitions to white with decreasing crystal size. Although odorless, lithium fluoride has a bitter-saline taste. Its structure is analogous to ...
, lithium hydroxide
Lithium hydroxide is an inorganic compound with the formula LiOH. It can exist as anhydrous or hydrated, and both forms are white hygroscopic solids. They are soluble in water and slightly soluble in ethanol. Both are available commercially. While ...
, lithium amide
Lithium amide or lithium azanide is an inorganic compound with the chemical formula . It is a white solid with a tetragonal crystal structure. Lithium amide can be made by treating lithium metal with liquid ammonia:
:
Other lithium amides
The con ...
, and methyllithium
Methyllithium is the simplest organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in ...
. The gap highlights the very low electronegativity of boron. Reaction of base with a borohydride R2BH does not result in deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.edu ...
to the boryl anion R2B− but to formation of the boryl anion R2B−H(base)+. This reaction product has a complete octet
Octet may refer to:
Music
* Octet (music), ensemble consisting of eight instruments or voices, or composition written for such an ensemble
** String octet, a piece of music written for eight string instruments
*** Octet (Mendelssohn), 1825 com ...
. Instead the boryl compound is prepared by reductive heterolysis of a boron-bromide bond by lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid e ...
metal. The new boryl lithium compound is very similar to and isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in the ...
with N-heterocyclic carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for ex ...
s. It is designed to benefit from aromatic stabilization (6-electron system counting the nitrogen lone pairs and an empty boron p-orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
, see structure A) and from kinetic stabilization from the bulky 2,6-diisopropylphenyl groups. X-ray diffraction
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angle ...
confirms sp2 hybridization
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to f ...
at boron and its nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions d ...
reaction with benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odo ...
gives further proof of the proposed structure.
Alkylideneboranes
Alkylideneboranes of the type RB=CRR with a boron – carbondouble bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
are rarely encountered. An example is borabenzene
Borabenzene is a hypothetical organoboron compound with the formula C5H5B. Unlike the related but highly stable benzene molecule, borabenzene would be electron-deficient. Related derivatives are the boratabenzene anions, including the parent 5H ...
. The parent compound is HB=CH2 which can be detected at low temperatures. A fairly stable derivative is CH3B=C(SiMe3)2 but is prone to cyclodimerisation.
NHC adducts of boron
NHCs and boranes form stable NHC borane adducts.Triethylborane
Triethylborane (TEB), also called triethylboron, is an organoborane (a compound with a B–C bond). It is a colorless pyrophoric liquid. Its chemical formula is or , abbreviated . It is soluble in organic solvents tetrahydrofuran and hexane.
P ...
adducts can be synthesised directly from the imidazolium salt and lithium triethylborohydride
Lithium triethylborohydride is the organoboron compound with the formula Li Et3 BH. Commonly referred to as LiTEBH or Superhydride, it is a powerful reducing agent used in organometallic and organic chemistry. It is a colorless or white liquid bu ...
. Members of this compound class are investigated for use as reagent or catalyst.
Diborenes
Chemical compounds with boron to borondouble bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
s are rare. In 2007 the first neutral diborene (RHB=BHR) was presented by Gregory Robinson of the University of Georgia
, mottoeng = "To teach, to serve, and to inquire into the nature of things.""To serve" was later added to the motto without changing the seal; the Latin motto directly translates as "To teach and to inquire into the nature of things."
, establ ...
. Each boron atom has a proton attached to it and each boron atom is coordinated to a NHC carbene. The parent structure with the additional carbene ligands is diborane(2)
Diborane(2) or diborene is a theoretical/hypothetical inorganic compound with the formula B2H2. Diborenes also refers to a series of molecules with a formula R:(BH)=(BH):R, where R is an organic group. B2H2 are unstable under ambient conditions. Th ...
.
:
A reported diboryne is based on similar chemistry.
Reactions
Organoboranes (R3B) and borates (R4B−, generated via addition of R− to R3B) possess boron–carbon bonds that are polarized toward carbon. Thus, the carbon attached to boron is nucleophilic, and in borates this property may be harnessed to transfer one of the R groups to an electrophilic center either inter- or (more often) intramolecularly. In the latter case, the nucleophilic R group is able to undergo 1,2-migration towards an electrophilic carbon attached to boron.Negishi, E.-i. ''J. Organometal. Chem.'' 1976, ''108'', 281. The resulting reorganized borane can then be oxidized or subjected to protonolysis to afford organic products. Examples covered in this article are shown below. Hydroboration of alkenes or alkynes is an efficient method for the generation of boranes; however, the use of borane (BH3) or borane equivalents leads to the conversion of only 33% of the starting olefin to product after oxidation or protonolysis—the remaining olefin is incorporated into boron-containing byproducts. The use of a stoichiometric amount of 9-borabicyclo .3.1onane (9-BBN) as the hydroborating reagent provides a solution to this problem.
Hydroboration of alkenes or alkynes is an efficient method for the generation of boranes; however, the use of borane (BH3) or borane equivalents leads to the conversion of only 33% of the starting olefin to product after oxidation or protonolysis—the remaining olefin is incorporated into boron-containing byproducts. The use of a stoichiometric amount of 9-borabicyclo .3.1onane (9-BBN) as the hydroborating reagent provides a solution to this problem.
Hydroboration-oxidation
In organic synthesis the hydroboration reaction is taken further to generate otherfunctional groups
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
in the place of the boron group. The hydroboration-oxidation reaction offers a route to alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s by oxidation of the borane with hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6 ...
or to the carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
group with the stronger oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
chromium oxide Chromium oxide may refer to:
* Chromium(II) oxide, CrO
* Chromium(III) oxide, Cr2O3
* Chromium dioxide (chromium(IV) oxide), CrO2, which includes the hypothetical compound chromium(II) chromate
* Chromium trioxide (chromium(VI) oxide), CrO3
* Chro ...
.
Rearrangements
Carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
is found to react with trialkylboranes. What follows is a 1,2-rearrangement whereby an alkyl substituent migrates from boron to the carbon of the carbonyl group. Homologated primary alcohols result from the treatment of organoboranes with carbon monoxide and a hydride.

Allylboration
Asymmetric allylboration demonstrates another useful application of organoboranes in carbon–carbon bond formation. In this example from Nicolaou's synthesis of theepothilone
Epothilones are a class of potential cancer drugs. Like taxanes, they prevent cancer cells from dividing by interfering with tubulin, but in early trials, epothilones have better efficacy and milder adverse effects than taxanes.
, epothilones A t ...
s, asymmetric allylboration (using an allylborane derived from chiral alpha-pinene
α-Pinene is an organic compound of the terpene class, one of two isomers of pinene. It is an alkene and it contains a reactive four-membered ring. It is found in the oils of many species of many coniferous trees, notably the pine. It is also ...
) is used in conjunction with TBS protection and ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond ...
. Overall, this provides a two-carbon homologation sequence that delivers the required acetogenin
Acetogenins are a class of polyketide natural products found in plants of the family Annonaceae. They are characterized by linear 32- or 34-carbon chains containing oxygenated functional groups including hydroxyls, ketones, epoxides, tetrahydrof ...
sequence.
:
As reducing agent
Borane hydrides such as 9-BBN and L-selectride (lithium tri-sec-butylborohydride) arereducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are commonly reducing agents include the Earth me ...
s. An example of an asymmetric catalyst for carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
reductions is the CBS catalyst
The CBS catalyst or Corey–Bakshi–Shibata catalyst is an asymmetric catalyst derived from proline. It finds many uses in organic reactions such as the CBS reduction, Diels-Alder reactions and (3+2) cycloadditions. Proline, a naturally ...
. This catalyst is also based on boron, the purpose of which is coordination to the carbonyl oxygen atom.
Borates
Trialkylboranes, BR3, can be oxidized to the correspondingborate
A borate is any of several boron oxyanions, negative ions consisting of boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt with such anions, such as sodium metaborate, and disodium tetraborate . The name also ...
s, B(OR)3. One method for the determination of the amount of C-B bonds in a compound is by oxidation of R3B with trimethylamine oxide (Me3NO) to B(OR)3. The trimethylamine
Trimethylamine (TMA) is an organic compound with the formula N(CH3)3. It is a colorless, hygroscopic, and flammable tertiary amine. It is a gas at room temperature but is usually sold as a 40% solution in water. (It is also sold in pressurized ...
(Me3N) formed can then be titrated
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of quantitative chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant'' ...
.
Boronic acid
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, memb ...
s RB(OH)2 react with potassium bifluoride
Potassium bifluoride is the inorganic compound with the formula . This colourless salt consists of the potassium cation () and the bifluoride anion (). The salt is used as an etchant for glass. Sodium bifluoride is related and is also of commerc ...
K F2to form trifluoroborate salts K BF3ref name="vedejs" /> which are precursors to nucleophilic alkyl and aryl boron difluorides, ArBF2. The salts are more stable than the boronic acids themselves and used for instance in alkylation of certain aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
s:
:
Suzuki reaction and related reactions
Organoboron compounds also lend themselves totransmetalation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
reactions, especially with organopalladium Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the ...
compounds. This reaction type is exemplified in the Suzuki reaction
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, a ...
, which involves coupling of aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
- or vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl m ...
-boronic acid
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, memb ...
with an aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
- or vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl m ...
-halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
catalyzed by a palladium(0) complex,
This reaction is an important method for making carbon-carbon bonds.
Mechanism and stereochemistry
Boranes alone are generally not nucleophilic enough to transfer an alkyl group to an electrophilic center. However, after nucleophilic attack, the resulting borate is highly nucleophilic. If the nucleophile contains unsaturated functionality or a leaving group at the α position, one of the R groups attached to boron is able to migrate to the electrophilic α carbon (see equation (2) below). The propensity of an organic group to migrate depends on its ability to stabilize negative charge: alkynyl > aryl ≈ alkenyl > primary alkyl > secondary alkyl > tertiary alkyl. Migration takes place with retention of configuration at the migrating carbon and inversion of configuration at the migration terminus (provided it is sp3 hybridized). Bis(norbornyl)borane and 9-BBN are often used as "dummy" hydroboration reagents for this reason—only the R group derived from the hydroborated olefin is likely to migrate upon nucleophilic activation. α-Halo enolates are commonly used as nucleophiles in this context. After nucleophilic attack at boron, the resulting ketoboronate rearranges to a neutral enolborane. Upon protonolysis, a functionalized carbonyl compound results. The intermediate enolboranes may also be quenched with electrophiles.
α-Halo enolates are commonly used as nucleophiles in this context. After nucleophilic attack at boron, the resulting ketoboronate rearranges to a neutral enolborane. Upon protonolysis, a functionalized carbonyl compound results. The intermediate enolboranes may also be quenched with electrophiles.
 Alkynylboronates are versatile intermediates that can be converted to either ketones or olefins after simultaneous migration and attack of the alkyne on a separate electrophile. The electrophile and migrating group end up ''trans'' in the resulting alkenylborane. Protonolysis of this intermediate generates olefins, while oxidation leads to ketones after tautomerization.
Alkynylboronates are versatile intermediates that can be converted to either ketones or olefins after simultaneous migration and attack of the alkyne on a separate electrophile. The electrophile and migrating group end up ''trans'' in the resulting alkenylborane. Protonolysis of this intermediate generates olefins, while oxidation leads to ketones after tautomerization.

Scope and limitations of reactions
The scope of organoboranes and borates as reagents for organic synthesis is extremely wide. Reactions of organoboron compounds may produce alcohols, carbonyl compounds, halides, peroxides, amines, and other functionality depending on other starting materials employed and reaction conditions. This section covers a small subset of these methods, focusing on the synthesis of alcohols, carbonyl compounds, and halides. Alcohol synthesis from organoboranes and borates relies on either nucleophilic group transfer to a carbonyl group or oxidation of an intermediate organoborane. Homologated primary alcohols result from the treatment of organoboranes with carbon monoxide and a hydride. Tertiary alcohols with two identical groups attached to the alcohol carbon may be synthesized through a double migration reaction of alkynylborates in the presence of acid.Midland, M. M.; Brown, H. C. ''J. Org. Chem.'' 1975, ''40'', 2845. Use of a single equivalent of acid and oxidation or protonolysis leads to ketones or olefins, respectively (see Mechanism and Stereochemistry section above).
Tertiary alcohols with two identical groups attached to the alcohol carbon may be synthesized through a double migration reaction of alkynylborates in the presence of acid.Midland, M. M.; Brown, H. C. ''J. Org. Chem.'' 1975, ''40'', 2845. Use of a single equivalent of acid and oxidation or protonolysis leads to ketones or olefins, respectively (see Mechanism and Stereochemistry section above).
 Acylation of borates is possible in the presence of an acyl halide. Here, the borate was generated from tri(cyclopentyl)borane and phenyllithium; the three cyclopentyl groups are serving as "dummy" groups and do not migrate to a significant amount.
Acylation of borates is possible in the presence of an acyl halide. Here, the borate was generated from tri(cyclopentyl)borane and phenyllithium; the three cyclopentyl groups are serving as "dummy" groups and do not migrate to a significant amount.
 Treatment of trialkylboranes with α-halo enolates leads to functionalized ketones.Brown, H. C.; Rogi, M. M.; Nambu, H.; Rathke, M. W. ''J. Am. Chem. Soc.'' 1969, ''91'', 2147. Because the migration is stereospecific (retentive with respect to the migrating group and invertive at the α carbon), this method provides a means for the synthesis of enantiopure α-alkyl or -aryl ketones.
Treatment of trialkylboranes with α-halo enolates leads to functionalized ketones.Brown, H. C.; Rogi, M. M.; Nambu, H.; Rathke, M. W. ''J. Am. Chem. Soc.'' 1969, ''91'', 2147. Because the migration is stereospecific (retentive with respect to the migrating group and invertive at the α carbon), this method provides a means for the synthesis of enantiopure α-alkyl or -aryl ketones.
 α-Halo ester enolates also add to boranes to eventually afford α-functionalized products; however, yields are slightly lower. Diazoesters and diazoketones may also be used in this context without the requirement for external base. α,α'-Dialo enolates react with boranes to form α-halo carbonyl compounds that can be further functionalized at the α position.
α-Halo ester enolates also add to boranes to eventually afford α-functionalized products; however, yields are slightly lower. Diazoesters and diazoketones may also be used in this context without the requirement for external base. α,α'-Dialo enolates react with boranes to form α-halo carbonyl compounds that can be further functionalized at the α position.
 Halides may be synthesized from organoboranes by activating with hydroxide or alkoxide and treatment with X2. Two of the three alkyl groups attached to the borane may be converted to halide in the presence of excess base, but the use of disiamylborane as the hydroborating reagent permits the selective halogenation of only the hydroborated olefin.
Halides may be synthesized from organoboranes by activating with hydroxide or alkoxide and treatment with X2. Two of the three alkyl groups attached to the borane may be converted to halide in the presence of excess base, but the use of disiamylborane as the hydroborating reagent permits the selective halogenation of only the hydroborated olefin.
 Treatment of an alkenylborane with iodine or bromine leads to migration of one of the organic groups attached to boron. Alkynyl groups migrate selectively, forming enynes after treatment with sodium acetate and hydrogen peroxide.Negishi, E.-i.; Lew, G.; Yoshida, T. ''Chem. Commun.'' 1973, 874.
Treatment of an alkenylborane with iodine or bromine leads to migration of one of the organic groups attached to boron. Alkynyl groups migrate selectively, forming enynes after treatment with sodium acetate and hydrogen peroxide.Negishi, E.-i.; Lew, G.; Yoshida, T. ''Chem. Commun.'' 1973, 874.

Other uses
TEB –Triethylborane
Triethylborane (TEB), also called triethylboron, is an organoborane (a compound with a B–C bond). It is a colorless pyrophoric liquid. Its chemical formula is or , abbreviated . It is soluble in organic solvents tetrahydrofuran and hexane.
P ...
was used to ignite the JP-7 fuel of the Pratt & Whitney J58
The Pratt & Whitney J58 (company designation JT11D-20) is an American jet engine that powered the Lockheed A-12, and subsequently the YF-12 and the SR-71 aircraft. It was an afterburning turbojet engine with a unique compressor bleed to the a ...
variable cycle engines powering the Lockheed SR-71 Blackbird
The Lockheed SR-71 "Blackbird" is a long-range, high-altitude, Mach 3+ strategic reconnaissance aircraft developed and manufactured by the American aerospace company Lockheed Corporation. It was operated by the United States Air Force ...
.
Notes
References
{{ChemicalBondsToCarbon