naloxone on:
[Wikipedia]
[Google]
[Amazon]
Naloxone, sold under the brand name Narcan among others, is an
 Naloxone is useful in treating both acute
Naloxone is useful in treating both acute

 In a survey of US laypersons in December 2021, most people believed the scientifically supported idea that trained bystanders can reverse overdoses with naloxone.
A survey of US naloxone prescription programs in 2010 revealed that 21 out of 48 programs reported challenges in obtaining naloxone in the months leading up to the survey, due mainly to either cost increases that outstripped allocated funding or the suppliers' inability to fill orders. The approximate cost of a 1ml ampoule of naloxone in the US is estimated to be significantly higher than in most other countries.
Take-home naloxone programs for people who use opioids are underway in many North American cities. CDC estimates that the US programs for drug users and their caregivers prescribing take-home doses of naloxone and training on its use prevented 10,000 opioid overdose deaths by 2014.
In Australia, some forms of naloxone are available "over the counter" in pharmacies free without a prescription under the Take Home Naloxone programme. It comes in single-use filled syringe form similar to law enforcement kits as well as nasal sprays. In 2024, those with a prescription can purchase five doses for around AU$32 or just over AU$6 per dose.
In Alberta, in addition to pharmacy distribution, take-home naloxone kits are available and distributed in most drug treatment or rehabilitation centers.
In the European Union, take home naloxone pilots were launched in the Channel Islands and in Berlin in the late 1990s. In 2008, the Welsh Assembly government announced its intention to establish demonstration sites for take-home naloxone, and in 2010, Scotland instituted a national naloxone program. Inspired by North American and European efforts, non-governmental organizations running programs to train drug users as overdose responders and supply them with naloxone are now operational in Russia, Ukraine, Georgia, Kazakhstan, Tajikistan, Afghanistan, China, Vietnam, and Thailand.
In 2018, a maker of naloxone announced it would provide a free kit including two doses of the nasal spray, as well as educational materials, to each of the 16,568 public libraries and 2,700 YMCAs in the U.S.
In 2025, an American start-up released a keychain case to make naloxone more immediately accessible in emergencies.
In April 2025, the city of Nashville, Tennessee introduced its first naloxone vending machine at a Twice Daily gas station on West End Avenue. This initiative, a collaboration between the Metro Nashville Health Department, Fund Recovery, and Twice Daily, aims to provide free access to naloxone, an opioid overdose-reversing medication. Within five weeks of installation, the machine dispensed over 2,200 doses, significantly surpassing initial expectations. Encouraged by this success, the health department plans to install three additional machines across Davidson County within 90 days, targeting areas with the highest overdose rates. The program is funded by opioid settlement money and underscores the importance of community partnerships in expanding access to life-saving interventions.
In a survey of US laypersons in December 2021, most people believed the scientifically supported idea that trained bystanders can reverse overdoses with naloxone.
A survey of US naloxone prescription programs in 2010 revealed that 21 out of 48 programs reported challenges in obtaining naloxone in the months leading up to the survey, due mainly to either cost increases that outstripped allocated funding or the suppliers' inability to fill orders. The approximate cost of a 1ml ampoule of naloxone in the US is estimated to be significantly higher than in most other countries.
Take-home naloxone programs for people who use opioids are underway in many North American cities. CDC estimates that the US programs for drug users and their caregivers prescribing take-home doses of naloxone and training on its use prevented 10,000 opioid overdose deaths by 2014.
In Australia, some forms of naloxone are available "over the counter" in pharmacies free without a prescription under the Take Home Naloxone programme. It comes in single-use filled syringe form similar to law enforcement kits as well as nasal sprays. In 2024, those with a prescription can purchase five doses for around AU$32 or just over AU$6 per dose.
In Alberta, in addition to pharmacy distribution, take-home naloxone kits are available and distributed in most drug treatment or rehabilitation centers.
In the European Union, take home naloxone pilots were launched in the Channel Islands and in Berlin in the late 1990s. In 2008, the Welsh Assembly government announced its intention to establish demonstration sites for take-home naloxone, and in 2010, Scotland instituted a national naloxone program. Inspired by North American and European efforts, non-governmental organizations running programs to train drug users as overdose responders and supply them with naloxone are now operational in Russia, Ukraine, Georgia, Kazakhstan, Tajikistan, Afghanistan, China, Vietnam, and Thailand.
In 2018, a maker of naloxone announced it would provide a free kit including two doses of the nasal spray, as well as educational materials, to each of the 16,568 public libraries and 2,700 YMCAs in the U.S.
In 2025, an American start-up released a keychain case to make naloxone more immediately accessible in emergencies.
In April 2025, the city of Nashville, Tennessee introduced its first naloxone vending machine at a Twice Daily gas station on West End Avenue. This initiative, a collaboration between the Metro Nashville Health Department, Fund Recovery, and Twice Daily, aims to provide free access to naloxone, an opioid overdose-reversing medication. Within five weeks of installation, the machine dispensed over 2,200 doses, significantly surpassing initial expectations. Encouraged by this success, the health department plans to install three additional machines across Davidson County within 90 days, targeting areas with the highest overdose rates. The program is funded by opioid settlement money and underscores the importance of community partnerships in expanding access to life-saving interventions.
opioid antagonist
An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist that acts on one or more of the opioid receptors.
Naloxone and naltrexone are commonly used opioid antagonist drugs which are competitive antagonists that bind to ...
, a medication used to reverse or reduce the effects of opioids
Opioids are a class of Drug, drugs that derive from, or mimic, natural substances found in the Papaver somniferum, opium poppy plant. Opioids work on opioid receptors in the brain and other organs to produce a variety of morphine-like effects, ...
. For example, it is used to restore breathing after an opioid overdose
An opioid overdose is toxicity due to excessive consumption of opioids, such as morphine, codeine, heroin, fentanyl, tramadol, and methadone. This preventable pathology can be fatal if it leads to respiratory depression, a lethal conditio ...
. Effects begin within two minutes when given intravenously
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutr ...
, five minutes when injected into a muscle, and ten minutes as a nasal spray
Nasal sprays are used to deliver medications Route of administration#Local, locally in the nasal cavities or systemic administration, systemically. They are used locally for conditions such as nasal congestion and allergic rhinitis. In some sit ...
. Naloxone blocks the effects of opioids for 30 to 90 minutes.
Administration to opioid-dependent individuals may cause symptoms of opioid withdrawal, including restlessness, agitation, nausea, vomiting, a fast heart rate, and sweating. To prevent this, small doses every few minutes can be given until the desired effect is reached. In those with previous heart disease or taking medications that negatively affect the heart, further heart problems have occurred. It appears to be safe in pregnancy, after having been given to a limited number of women. Naloxone is a non-selective and competitive
Competition is a rivalry where two or more parties strive for a common goal which cannot be shared: where one's gain is the other's loss (an example of which is a zero-sum game). Competition can arise between entities such as organisms, indi ...
opioid receptor antagonist. It reverses the depression of the central nervous system and respiratory system caused by opioids.
Naloxone was patented in 1961 and approved for opioid overdose in the United States in 1971. It is on the World Health Organization's List of Essential Medicines
The WHO Model List of Essential Medicines (aka Essential Medicines List or EML), published by the World Health Organization (WHO), contains the medications considered to be most effective and safe to meet the most important needs in a health s ...
.
Medical uses
Opioid overdose
 Naloxone is useful in treating both acute
Naloxone is useful in treating both acute opioid overdose
An opioid overdose is toxicity due to excessive consumption of opioids, such as morphine, codeine, heroin, fentanyl, tramadol, and methadone. This preventable pathology can be fatal if it leads to respiratory depression, a lethal conditio ...
and respiratory or mental depression due to opioids. Whether it is useful in those in cardiac arrest
Cardiac arrest (also known as sudden cardiac arrest CA is when the heart suddenly and unexpectedly stops beating. When the heart stops beating, blood cannot properly Circulatory system, circulate around the body and the blood flow to the ...
due to an opioid overdose is unclear.
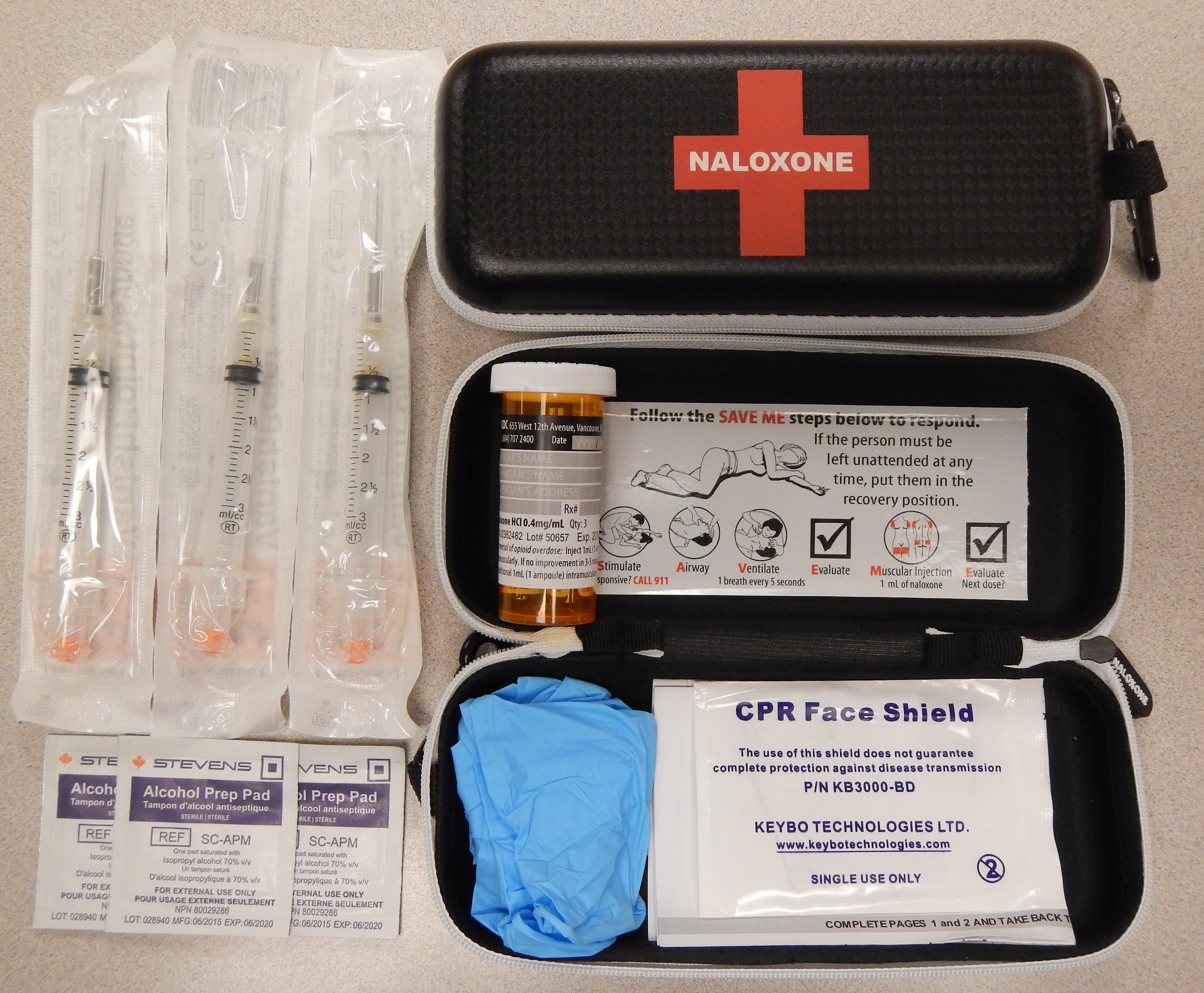
It is included as a part of emergency overdose response kits distributed to heroin
Heroin, also known as diacetylmorphine and diamorphine among other names, is a morphinan opioid substance synthesized from the Opium, dried latex of the Papaver somniferum, opium poppy; it is mainly used as a recreational drug for its eupho ...
, fentanyl
Fentanyl is a highly potent synthetic piperidine opioid primarily used as an analgesic (pain medication). It is 30 to 50 times more Potency (pharmacology), potent than heroin and 50 to 100 times more potent than morphine. Its primary Medici ...
, and other opioid drug users, and to emergency responders. This has been shown to reduce rates of deaths due to overdose. A prescription for naloxone is recommended if a person is on a high dose of opioid (>100mg of morphine
Morphine, formerly also called morphia, is an opiate that is found naturally in opium, a dark brown resin produced by drying the latex of opium poppies (''Papaver somniferum''). It is mainly used as an analgesic (pain medication). There are ...
equivalence/day), is prescribed any dose of opioid accompanied by a benzodiazepine
Benzodiazepines (BZD, BDZ, BZs), colloquially known as "benzos", are a class of central nervous system (CNS) depressant, depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed t ...
, or is suspected or known to use opioids nonmedically. Prescribing naloxone should be accompanied by standard education that includes preventing, identifying, and responding to an overdose; rescue breathing; and calling emergency services.
Distribution of naloxone to individuals likely to encounter people who overdose is one aspect of harm reduction
Harm reduction, or harm minimization, refers to a range of intentional practices and public health policies designed to lessen the negative social and/or physical consequences associated with various human behaviors, both legal and illegal. H ...
strategies.
However, with opioids that have longer half-lives, respiratory depression returns after naloxone has worn off; therefore, adequate dosing and continuous monitoring may be necessary.
Clonidine overdose
Naloxone can also be used as an antidote in an overdose ofclonidine
Clonidine, sold under the brand name Catapres among others, is an α2A-adrenergic receptor agonist medication used to treat high blood pressure, attention deficit hyperactivity disorder (ADHD), drug withdrawal (e.g., alcohol, opioids, or nic ...
, a medication that lowers blood pressure. Clonidine overdoses are of special relevance for children, in whom even small doses can cause significant harm. However, there is controversy regarding naloxone's efficacy in treating the symptoms of clonidine overdose, namely slow heart rate, low blood pressure, and confusion/somnolence. Case reports that used doses of 0.1mg/kg (maximum of 2mg/dose) repeated every 1–2 minutes (10mg total dose) have shown inconsistent benefit. As the doses used throughout the literature vary, it is difficult to form a conclusion regarding the benefit of naloxone in this setting. The mechanism for naloxone's proposed benefit in clonidine overdose is unclear. Still, it has been suggested that endogenous opioid receptors mediate the sympathetic nervous system
The sympathetic nervous system (SNS or SANS, sympathetic autonomic nervous system, to differentiate it from the somatic nervous system) is one of the three divisions of the autonomic nervous system, the others being the parasympathetic nervous sy ...
in the brain and elsewhere in the body.
Preventing recreational opioid use
Naloxone is poorly absorbed when taken orally or sublingually, so it is often combined with several oral or sublingual opioid preparations, including buprenorphine and pentazocine, so that when swallowed or taken sublingually, only the non-naloxone opioid has an effect. However, if the combination is injected (such as by dissolving a pill or sublingual strip in water), the naloxone is believed to block the effect of the other opioid. This combination is used to prevent non-medical use. However,SAMHSA
The Substance Abuse and Mental Health Services Administration (SAMHSA; pronounced ) is a branch of the U.S. Department of Health and Human Services (HHS). SAMHSA is charged with improving the quality and availability of treatment and rehabilitat ...
's clinical guidelines state that if the combination of buprenorphine and naloxone is injected by a regular user of buprenorphine or buprenorphine/naloxone, then the buprenorphine would still produce an agonist effect but the naloxone would fail to produce an antagonist effect. This is because the amount of naloxone that would be required to block the buprenorphine after injection is much larger than the amount that is contained in buprenorphine/naloxone (Suboxone) pills and strips. If someone who is not physically dependent on opioids were to inject the buprenorphine/naloxone combination, then the effects of the buprenorphine may at most be slightly lessened, but the individual would still be expected to experience euphoric effects.
Other uses
A 2003 meta-analysis of existing research showed naloxone to improve blood flow in patients with shock, including septic, cardiogenic, hemorrhagic, or spinal shock, but could not determine if this reduced patient deaths. Oral naloxone has been used for opioid-induced constipation (OIC). A 2018 meta-analysis cites 5 studies that tests it for this purpose. It found that medical treatment for OIC can be more efficacious than placebo, but did not look into the effect of individual treatments such as naloxone. As a result, no conclusion can be drawn from the study on naloxone's effectiveness for OIC.Special populations
Pregnancy and breastfeeding
Whether naloxone is excreted inbreast milk
Breast milk (sometimes spelled as breastmilk) or mother's milk is milk produced by the mammary glands in the breasts of women. Breast milk is the primary source of nutrition for newborn infants, comprising fats, proteins, carbohydrates, and a var ...
is unknown, however, it is not orally bioavailable and therefore is unlikely to affect a breastfeeding
Breastfeeding, also known as nursing, is the process where breast milk is fed to a child. Infants may suck the milk directly from the breast, or milk may be extracted with a Breast pump, pump and then fed to the infant. The World Health Orga ...
infant.
Children
Naloxone can be used on infants who were exposed to intrauterine opiates administered to mothers during delivery. However, there is insufficient evidence for the use of naloxone to lower cardiorespiratory and neurological depression in these infants. Infants exposed to high concentrations of opiates during pregnancy may have CNS damage in the setting of perinatal asphyxia. Naloxone has been studied to improve outcomes in this population, however the evidence is currently weak. Intravenous, intramuscular, or subcutaneous administration of naloxone can be given to children and neonates to reverse opiate effects. TheAmerican Academy of Pediatrics
The American Academy of Pediatrics (AAP) is the largest professional association of pediatricians in the United States. It is headquartered in Itasca, Illinois, and maintains an office in Washington, D.C. The AAP has published hundreds of poli ...
recommends only intravenous administration as the other two forms can cause unpredictable absorption. After a dose is given, the child should be monitored for at least 24 hours. For children with low blood pressure due to septic shock
Septic shock is a potentially fatal medical condition that occurs when sepsis, which is organ injury or damage in response to infection, leads to dangerously low blood pressure and abnormalities in cellular metabolism. The Third International C ...
, naloxone safety and effectiveness are not established.
Geriatric use
For patients 65 years and older, it is unclear if there is a difference in response. However, older people often have decreased liver and kidney function which may lead to an increased level of naloxone in their body.Available forms
Intravenous
In hospital settings, naloxone is injectedintravenously
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutr ...
, with an onset of 1–2 minutes and a duration of up to 45 minutes.
Intramuscular or subcutaneous
Naloxone can also be administered via intramuscular orsubcutaneous injection
Subcutaneous administration is the insertion of medications beneath the skin either by injection or infusion.
A subcutaneous injection is administered as a bolus (medicine), bolus into the subcutis, the layer of skin directly below the dermis and ...
. The onset of naloxone provided through this route is 2 to 5 minutes with a duration of around 30–120min. Naloxone administered intramuscularly are provided through pre-filled syringes, vials, and auto-injector. A hand-held auto-injector is pocket-sized and can be used in non-medical settings such as in the home. It is designed for use by laypersons, including family members and caregivers of opioid users at risk for an opioid emergency, such as an overdose. According to the FDA's National Drug Code Directory, a generic version of the auto-injector began to be marketed at the end of 2019.
Intranasal
Narcan nasal spray was approved in the US in 2015 and is the first FDA-approved nasal spray for emergency treatment or suspected overdose. It was developed in a partnership between LightLake Therapeutics and theNational Institute on Drug Abuse
The National Institute on Drug Abuse (NIDA) is a United States federal government research institute whose mission is to "advance science on the causes and consequences of drug use and addiction and to apply that knowledge to improve individual ...
. The approval process was fast-tracked. A generic version of the nasal spray was approved in the United States in 2019, though did not come to market until 2021.
In 2021, the FDA approved Kloxxado, an 8mg dose of intranasal naloxone developed by Hikma Pharmaceuticals. Citing the frequent need for multiple 4mg doses of Narcan to successfully reverse overdose, packs of Kloxxado Nasal Spray contain two pre-packaged nasal spray devices, each containing 8mg of naloxone.
However, a wedge device (nasal atomizer) can also be attached to a syringe that may also be used to create a mist to deliver the drug to the nasalmucosa
A mucous membrane or mucosa is a membrane that lines various cavities in the body of an organism and covers the surface of internal organs. It consists of one or more layers of epithelial cells overlying a layer of loose connective tissue. It ...
. This is useful near facilities where many overdoses occur that already stock injectors.

Side effects
Administration of naloxone to somebody who has used opioids may cause rapid-onset opioid withdrawal. Naloxone has little to no effect if opioids are not present. In people with opioids in their system, it may cause increased sweating, nausea, restlessness, trembling, vomiting, flushing, and headache, and has in rare cases been associated with heart rhythm changes,seizures
A seizure is a sudden, brief disruption of brain activity caused by abnormal, excessive, or synchronous neuronal firing. Depending on the regions of the brain involved, seizures can lead to changes in movement, sensation, behavior, awareness, o ...
, and pulmonary edema
Pulmonary edema (British English: oedema), also known as pulmonary congestion, is excessive fluid accumulation in the tissue or air spaces (usually alveoli) of the lungs. This leads to impaired gas exchange, most often leading to shortness ...
.
Naloxone has been shown to block the action of pain-lowering endorphin
Endorphins (contracted from endogenous morphine) are peptides produced in the brain that block the perception of pain and increase feelings of wellbeing. They are produced and stored in the pituitary gland of the brain. Endorphins are endogeno ...
s the body produces naturally. These endorphins likely operate on the same opioid receptors that naloxone blocks. It is capable of blocking a placebo
A placebo ( ) can be roughly defined as a sham medical treatment. Common placebos include inert tablets (like sugar pills), inert injections (like saline), sham surgery, and other procedures.
Placebos are used in randomized clinical trials ...
pain-lowering response if the placebo is administered together with a hidden or blind injection of naloxone. Other studies have found that placebo alone can activate the body's μ-opioid endorphin system, delivering pain relief by the same receptor mechanism as morphine.
Naloxone should be used with caution in people with cardiovascular disease as well as those who are currently taking medications that could have adverse effects on the cardiovascular system such as causing low blood pressure, fluid accumulation in the lungs (pulmonary edema), and abnormal heart rhythms. There have been reports of abrupt reversals with opioid antagonists leading to pulmonary edema and ventricular fibrillation
Ventricular fibrillation (V-fib or VF) is an abnormal heart rhythm in which the Ventricle (heart), ventricles of the heart Fibrillation, quiver. It is due to disorganized electrical conduction system of the heart, electrical activity. Ventricula ...
.
Use of naloxone to treat people who have been using opioids recreationally may cause acute opioid withdrawal with distressing physiological symptoms such as shivering, tachycardia
Tachycardia, also called tachyarrhythmia, is a heart rate that exceeds the normal resting rate. In general, a resting heart rate over 100 beats per minute is accepted as tachycardia in adults. Heart rates above the resting rate may be normal ...
, and nausea; these in turn may lead to aggression and reluctance to receive further treatment.
Pharmacology
Pharmacodynamics
Naloxone is alipophilic
Lipophilicity (from Greek language, Greek λίπος "fat" and :wikt:φίλος, φίλος "friendly") is the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such compounds are c ...
compound that acts as a non-selective and competitive
Competition is a rivalry where two or more parties strive for a common goal which cannot be shared: where one's gain is the other's loss (an example of which is a zero-sum game). Competition can arise between entities such as organisms, indi ...
opioid receptor antagonist. The pharmacologically active isomer of naloxone is (−)-naloxone. Naloxone's binding affinity
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usuall ...
is highest for the μ-opioid receptor
The μ-opioid receptors (MOR) are a class of opioid receptors with a high affinity for enkephalins and beta-endorphin, but a low affinity for dynorphins. They are also referred to as μ(''mu'')-opioid peptide (MOP) receptors. The prototypical ...
(MOR), then the δ-opioid receptor
The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi alpha subunit, Gi/G0 and has enkephalins as it ...
(DOR), and lowest for the κ-opioid receptor
The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP for its ligand ketazocine, is a G protein-coupled receptor that in humans is encoded by the ''OPRK1'' gene. The KOR is coupled to the G protein Gi/G0 and is one of four re ...
(KOR); naloxone has negligible affinity for the nociceptin receptor.
If naloxone is administered in the absence of concomitant opioid use, no functional pharmacological activity occurs, except the inability of the body to combat pain naturally; since pure mu-opioid antagonists like naloxone and naltrexone
Naltrexone, sold under the brand name Revia among others, is a medication primarily used to manage alcohol use or opioid use disorder by reducing cravings and feelings of euphoria associated with substance use disorder. It has also been ...
block the effects of endorphins. In contrast to direct opiate agonists, which elicit opiate withdrawal symptoms when discontinued in opiate-tolerant people, no evidence indicates the development of tolerance or dependence on naloxone. The mechanism of action is not completely understood, but studies suggest it functions to produce withdrawal symptoms by competing for opioid receptors within the brain (a competitive antagonist, not a direct agonist), thereby preventing the action of both endogenous
Endogeny, in biology, refers to the property of originating or developing from within an organism, tissue, or cell.
For example, ''endogenous substances'', and ''endogenous processes'' are those that originate within a living system (e.g. an ...
and xenobiotic opioids on these receptors without directly producing any effects itself.
A single administration of naloxone at a relatively high dose of 2mg by intravenous injection
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutr ...
has been found to produce brain MOR blockade of 80% at 5minutes, 47% at 2hours, 44% at 4hours, and 8% at 8hours. A low dose (2μg/kg) produced brain MOR blockade of 42% at 5minutes, 36% at 2hours, 33% at 4hours, and 10% at 8hours. Intranasal administration of naloxone via nasal spray
Nasal sprays are used to deliver medications Route of administration#Local, locally in the nasal cavities or systemic administration, systemically. They are used locally for conditions such as nasal congestion and allergic rhinitis. In some sit ...
has likewise been found to rapidly occupy brain MORs, with peak occupancy occurring at 20minutes, peak occupancies of 67% at a dose of 2mg and 85% with 4mg, and an estimated half-life of occupancy disappearance of approximately 100minutes (1.67hours).
Pharmacokinetics
When administered parenterally (non-orally or non-rectally, e.g., intravenously or by injection), as is most common, naloxone has a rapid distribution throughout the body. The mean serum half-life has been shown to range from 30 to 81 minutes, shorter than the average half-life of some opiates, necessitating repeat dosing if opioid receptors must be stopped from triggering for an extended period. Naloxone is primarily metabolized by the liver. Its major metabolite is naloxone-3-glucuronide, which is excreted in the urine. For people with liver diseases such as alcoholic liver disease orhepatitis
Hepatitis is inflammation of the liver parenchyma, liver tissue. Some people or animals with hepatitis have no symptoms, whereas others develop yellow discoloration of the skin and whites of the eyes (jaundice), Anorexia (symptom), poor appetite ...
, naloxone usage has not been shown to increase serum liver enzyme levels.
Naloxone has low systemic bioavailability when taken by mouth due to hepatic first-pass metabolism, but it does block opioid receptors that are located in the intestine.
Chemistry
Naloxone, also known as N-allylnoroxymorphone or as 17-allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one, is a synthetic morphinanderivative
In mathematics, the derivative is a fundamental tool that quantifies the sensitivity to change of a function's output with respect to its input. The derivative of a function of a single variable at a chosen input value, when it exists, is t ...
and was derived from oxymorphone
Oxymorphone (sold under the brand names Numorphan and Opana among others) is a highly potent opioid analgesic indicated for treatment of severe pain. Pain relief after injection begins after about 5–10 minutes; after oral administration it ...
(14-hydroxydihydromorphinone), an opioid analgesic. Oxymorphone, in turn, was derived from morphine
Morphine, formerly also called morphia, is an opiate that is found naturally in opium, a dark brown resin produced by drying the latex of opium poppies (''Papaver somniferum''). It is mainly used as an analgesic (pain medication). There are ...
, an opioid analgesic and naturally occurring
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical ...
constituent of the opium poppy. Naloxone is a racemic mixture
In chemistry, a racemic mixture or racemate () is a mixture that has equal amounts (50:50) of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as r ...
of two enantiomer
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...
s, (–)-naloxone (levonaloxone) and (+)-naloxone (dextronaloxone), only the former of which is active at opioid receptors. The drug is highly lipophilic
Lipophilicity (from Greek language, Greek λίπος "fat" and :wikt:φίλος, φίλος "friendly") is the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such compounds are c ...
, allowing it to rapidly penetrate the brain and to achieve a far greater brain to serum ratio than that of morphine. Opioid antagonists related to naloxone include cyprodime, nalmefene
Nalmefene, sold under the brand name Revex among others, is a medication that is used in the treatment of opioid overdose and alcohol dependence. Nalmefene belongs to the class of opioid antagonists and can be taken by mouth, administered by ...
, nalodeine, naloxol, and naltrexone
Naltrexone, sold under the brand name Revia among others, is a medication primarily used to manage alcohol use or opioid use disorder by reducing cravings and feelings of euphoria associated with substance use disorder. It has also been ...
.
History
Naloxone was patented in 1961 by Mozes J. Lewenstein, Jack Fishman, and the company Sankyo. It was approved for opioid use disorder treatment in the United States in 1971.Society and culture
Misinformation
Naloxone has been subject to much inaccurate media reporting and manyurban legend
Urban legend (sometimes modern legend, urban myth, or simply legend) is a genre of folklore concerning stories about an unusual (usually scary) or humorous event that many people believe to be true but largely are not.
These legends can be e ...
s about it have become prevalent.
One such myth is that naloxone makes the recipient violent. Another is that events called "Lazarus parties" have taken place, in which people reportedly took fatal overdoses in anticipation of being treated with naloxone; in reality this was a fiction spread by the police. Yet another is the claim that people have indulged in "yo-yoing", whereby they would take naloxone and opioids simultaneously to enjoy an extreme "high" and subsequent revival; the idea is scientifically nonsensical.
Names
Naloxone is its international nonproprietary name,British Approved Name
A British Approved Name (BAN) is the official, non-proprietary, or generic name given to a pharmaceutical substance, as defined in the British Pharmacopoeia (BP). The BAN is also the official name used in some countries around the world, because ...
, Dénomination Commune Française, Denominazione Comune Italiana, and Japanese Accepted Name
A (JAN) is the official non-proprietary or generic name given to a pharmaceutical substance by the Government of Japan.
See also
* International Nonproprietary Name (INN)
* United States Adopted Name (USAN)
* British Approved Name (BAN)
* ...
, while naloxone hydrochloride is its United States Adopted Name and British Approved Name (Modified).
The patent
A patent is a type of intellectual property that gives its owner the legal right to exclude others from making, using, or selling an invention for a limited period of time in exchange for publishing an sufficiency of disclosure, enabling discl ...
has expired and it is available as a generic medication
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active ch ...
. Several formulations use patented dispensers (spray mechanisms or autoinjectors), and patent disputes over the generic forms of the nasal spray were litigated between 2016 and 2020 when a judge ruled in favor of Teva, the generic manufacturer. Teva announced entry of the first generic nasal spray formulation in December 2021. Brand names of naloxone include Narcan, Kloxxado, Nalone, Evzio, Prenoxad Injection, Narcanti, Narcotan, and Zimhi, among others.
Legal status and availability to law enforcement and emergency personnel
Naloxone (Nyxoid) was approved for use in the European Union in September 2017. In the United States, some nasal naloxone are legally available without a prescription. As of 2019, officials in 29 states had issued standing orders to enable licensed pharmacists to provide naloxone to patients without the individual first visiting a prescriber. Prescribers working with harm reduction or low threshold treatment programs have also issued standing orders to enable these organizations to distribute naloxone to their clients. A standing order, also referred to as a "non-patient specific prescription" is written by a physician, nurse or other prescriber to authorize medicine distribution outside the doctor-patient relationship. In the case of naloxone, these orders are meant to facilitate naloxone distribution to people using opioids, and their family members and friends. Over 200 naloxone distribution programs utilize licensed prescribers to distribute the drug through such orders, or through the authority of pharmacists (as with California's legal provision, AB1535). Laws and policies in many US jurisdictions have been changed to allow wider distribution of naloxone. In addition to laws or regulations permitting distribution of medicine to at-risk individuals and families, some 36 states have passed laws that provide naloxone prescribers with immunity against both civil and criminal liabilities. While paramedics in the US have carried naloxone for decades, law enforcement officers in many states throughout the country carry naloxone to reverse the effects of heroin overdoses when reaching the location before paramedics. As of 12 July 2015, law enforcement departments in 28 US states are allowed to or required to carry naloxone to quickly respond to opioid overdoses. Programs training fire personnel in opioid overdose response using naloxone have also shown promise in the US, and efforts to integrate opioid fatality prevention into emergency response have grown due to the US overdose crisis. Following the use of the nasal spray device by police officers on Staten Island in New York, an additional 20,000 police officers began carrying naloxone in mid-2014. The state's Office of the Attorney General provided US$1.2 million to supply nearly 20,000 kits. Police Commissioner William Bratton said: "Naloxone gives individuals a second chance to get help".Emergency Medical Service
Emergency medical services (EMS), also known as ambulance services, pre-hospital care or paramedic services, are emergency services that provide urgent pre-hospital treatment and stabilisation for serious illness and injuries and transport to ...
Providers (EMS) routinely administer naloxone, except where basic Emergency Medical Technicians are prohibited by policy or by state law. In efforts to encourage citizens to seek help for possible opioid overdoses, many states have adopted Good Samaritan laws that provide immunity against certain criminal liabilities for anybody who, in good faith, seeks emergency medical care for either themselves or someone around them who may be experiencing an opioid overdose.
States including Vermont and Virginia have developed programs that mandate the prescription of naloxone when a prescription has exceeded a certain level of morphine milliequivalents per day as preventative measures against overdose. Healthcare institution-based naloxone prescription programs have also helped reduce rates of opioid overdose in North Carolina
North Carolina ( ) is a U.S. state, state in the Southeastern United States, Southeastern region of the United States. It is bordered by Virginia to the north, the Atlantic Ocean to the east, South Carolina to the south, Georgia (U.S. stat ...
, and have been replicated in the US military.
In Canada, naloxone single-use syringe kits are distributed and available at various clinics and emergency rooms. Alberta Health Services
Alberta Health Services (AHS) is the single Health regions of Canada, health authority for the Provinces and territories of Canada, Canadian province of Alberta and the "largest integrated provincial health care system" in Canada. Headquartered ...
is increasing the distribution points for naloxone kits at all emergency rooms, and various pharmacies and clinics province-wide. All Edmonton Police Service and Calgary Police Service patrol cars carry an emergency single-use naloxone syringe kit. Some Royal Canadian Mounted Police
The Royal Canadian Mounted Police (RCMP; , GRC) is the Law enforcement in Canada, national police service of Canada. The RCMP is an agency of the Government of Canada; it also provides police services under contract to 11 Provinces and terri ...
patrol vehicles also carry the drug, occasionally in excess to help distribute naloxone among users and concerned family/friends. Nurses, paramedics, medical technicians, and emergency medical responders can also prescribe and distribute the drug. As of February 2016, pharmacies across Alberta
Alberta is a Provinces and territories of Canada, province in Canada. It is a part of Western Canada and is one of the three Canadian Prairies, prairie provinces. Alberta is bordered by British Columbia to its west, Saskatchewan to its east, t ...
and some other Canadian jurisdictions are allowed to distribute single-use take-home naloxone kits or prescribe the drug to people using opioids.
Following Alberta Health Services, Health Canada
Health Canada (HC; )Health Canada is the applied title under the Federal Identity Program; the legal title is Department of Health (). is the Structure of the Canadian federal government#Departments, with subsidiary units, department of the Gove ...
reviewed the prescription-only status of naloxone, resulting in plans to remove it in 2016, making naloxone more accessible. Due to the rising number of drug deaths across the country, Health Canada proposed a change to make naloxone more widely available to Canadians in support of efforts to address the growing number of opioid overdoses. In March 2016, Health Canada did change the prescription status of naloxone, as "pharmacies are now able to proactively give out naloxone to those who might experience or witness an opioid overdose."
Community access
 In a survey of US laypersons in December 2021, most people believed the scientifically supported idea that trained bystanders can reverse overdoses with naloxone.
A survey of US naloxone prescription programs in 2010 revealed that 21 out of 48 programs reported challenges in obtaining naloxone in the months leading up to the survey, due mainly to either cost increases that outstripped allocated funding or the suppliers' inability to fill orders. The approximate cost of a 1ml ampoule of naloxone in the US is estimated to be significantly higher than in most other countries.
Take-home naloxone programs for people who use opioids are underway in many North American cities. CDC estimates that the US programs for drug users and their caregivers prescribing take-home doses of naloxone and training on its use prevented 10,000 opioid overdose deaths by 2014.
In Australia, some forms of naloxone are available "over the counter" in pharmacies free without a prescription under the Take Home Naloxone programme. It comes in single-use filled syringe form similar to law enforcement kits as well as nasal sprays. In 2024, those with a prescription can purchase five doses for around AU$32 or just over AU$6 per dose.
In Alberta, in addition to pharmacy distribution, take-home naloxone kits are available and distributed in most drug treatment or rehabilitation centers.
In the European Union, take home naloxone pilots were launched in the Channel Islands and in Berlin in the late 1990s. In 2008, the Welsh Assembly government announced its intention to establish demonstration sites for take-home naloxone, and in 2010, Scotland instituted a national naloxone program. Inspired by North American and European efforts, non-governmental organizations running programs to train drug users as overdose responders and supply them with naloxone are now operational in Russia, Ukraine, Georgia, Kazakhstan, Tajikistan, Afghanistan, China, Vietnam, and Thailand.
In 2018, a maker of naloxone announced it would provide a free kit including two doses of the nasal spray, as well as educational materials, to each of the 16,568 public libraries and 2,700 YMCAs in the U.S.
In 2025, an American start-up released a keychain case to make naloxone more immediately accessible in emergencies.
In April 2025, the city of Nashville, Tennessee introduced its first naloxone vending machine at a Twice Daily gas station on West End Avenue. This initiative, a collaboration between the Metro Nashville Health Department, Fund Recovery, and Twice Daily, aims to provide free access to naloxone, an opioid overdose-reversing medication. Within five weeks of installation, the machine dispensed over 2,200 doses, significantly surpassing initial expectations. Encouraged by this success, the health department plans to install three additional machines across Davidson County within 90 days, targeting areas with the highest overdose rates. The program is funded by opioid settlement money and underscores the importance of community partnerships in expanding access to life-saving interventions.
In a survey of US laypersons in December 2021, most people believed the scientifically supported idea that trained bystanders can reverse overdoses with naloxone.
A survey of US naloxone prescription programs in 2010 revealed that 21 out of 48 programs reported challenges in obtaining naloxone in the months leading up to the survey, due mainly to either cost increases that outstripped allocated funding or the suppliers' inability to fill orders. The approximate cost of a 1ml ampoule of naloxone in the US is estimated to be significantly higher than in most other countries.
Take-home naloxone programs for people who use opioids are underway in many North American cities. CDC estimates that the US programs for drug users and their caregivers prescribing take-home doses of naloxone and training on its use prevented 10,000 opioid overdose deaths by 2014.
In Australia, some forms of naloxone are available "over the counter" in pharmacies free without a prescription under the Take Home Naloxone programme. It comes in single-use filled syringe form similar to law enforcement kits as well as nasal sprays. In 2024, those with a prescription can purchase five doses for around AU$32 or just over AU$6 per dose.
In Alberta, in addition to pharmacy distribution, take-home naloxone kits are available and distributed in most drug treatment or rehabilitation centers.
In the European Union, take home naloxone pilots were launched in the Channel Islands and in Berlin in the late 1990s. In 2008, the Welsh Assembly government announced its intention to establish demonstration sites for take-home naloxone, and in 2010, Scotland instituted a national naloxone program. Inspired by North American and European efforts, non-governmental organizations running programs to train drug users as overdose responders and supply them with naloxone are now operational in Russia, Ukraine, Georgia, Kazakhstan, Tajikistan, Afghanistan, China, Vietnam, and Thailand.
In 2018, a maker of naloxone announced it would provide a free kit including two doses of the nasal spray, as well as educational materials, to each of the 16,568 public libraries and 2,700 YMCAs in the U.S.
In 2025, an American start-up released a keychain case to make naloxone more immediately accessible in emergencies.
In April 2025, the city of Nashville, Tennessee introduced its first naloxone vending machine at a Twice Daily gas station on West End Avenue. This initiative, a collaboration between the Metro Nashville Health Department, Fund Recovery, and Twice Daily, aims to provide free access to naloxone, an opioid overdose-reversing medication. Within five weeks of installation, the machine dispensed over 2,200 doses, significantly surpassing initial expectations. Encouraged by this success, the health department plans to install three additional machines across Davidson County within 90 days, targeting areas with the highest overdose rates. The program is funded by opioid settlement money and underscores the importance of community partnerships in expanding access to life-saving interventions.
Veterinary use
Naloxone is used to reverse pure μ-opioid receptor agonists and for opioid reversal during cardiopulmonary arrest in cats and dogs. This is often given intravenously but instranasal administration works in the dog at higher doses. Renarcotisation can occur with naloxone administration, especially with morphinans. Naxlone administration has been reported to result in altered mental status andblepharospasm
Blepharospasm is a neurological disorder characterized by intermittent, involuntary spasms and contractions of the orbicularis oculi muscle, orbicularis oculi (eyelid) muscles around both eyes. These result in abnormal twitching or blinking, an ...
. Aetiology
Etiology (; alternatively spelled aetiology or ætiology) is the study of causation or origination. The word is derived from the Greek word ''()'', meaning "giving a reason for" (). More completely, etiology is the study of the causes, origin ...
of this is unknown but could be due to either withdrawal, systemic hypertension, or rapid changes to the cerebral vascular tone. One study in horses reported that naloxone administration reduced increases in right ventricular pressure and heart rate caused by endotoxic shock.
References
Further reading
*External links
* * {{Authority control Allylamines Antidotes Chemical substances for emergency medicine 4,5-Epoxymorphinans GABAA receptor negative allosteric modulators Kappa-opioid receptor antagonists Ketones Mu-opioid receptor antagonists Phenol ethers Sigma antagonists Tertiary alcohols Wikipedia medicine articles ready to translate World Health Organization essential medicines