Mendelevium on:
[Wikipedia]
[Google]
[Amazon]
Mendelevium is a
 Mendelevium was the ninth transuranic element to be synthesized. It was first synthesized by Albert Ghiorso, Glenn T. Seaborg,
Mendelevium was the ninth transuranic element to be synthesized. It was first synthesized by Albert Ghiorso, Glenn T. Seaborg,  On the day of discovery, 19 February, alpha irradiation of the einsteinium target occurred in three three-hour sessions. The cyclotron was in the
On the day of discovery, 19 February, alpha irradiation of the einsteinium target occurred in three three-hour sessions. The cyclotron was in the
 In the
In the
Los Alamos National Laboratory – Mendelevium
at '' The Periodic Table of Videos'' (University of Nottingham)
Environmental Chemistry – Md info
{{Authority control Chemical elements Chemical elements with face-centered cubic structure Actinides Synthetic elements
synthetic element
A synthetic element is one of 24 known chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Md ( formerly Mv) and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
101. A metallic radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
transuranium element in the actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
series, it is the first element by atomic number that currently cannot be produced in macroscopic quantities by neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
bombardment of lighter elements. It is the third-to-last actinide and the ninth transuranic element. It can only be produced in particle accelerator
A particle accelerator is a machine that uses electromagnetic fields to propel charged particles to very high speeds and energies, and to contain them in well-defined beams.
Large accelerators are used for fundamental research in particle ...
s by bombarding lighter elements with charged particles. Seventeen isotopes
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass numbers ...
are known; the most stable is 258Md with half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
51 days; however, the shorter-lived 256Md (half-life 1.17 hour
An hour (symbol: h; also abbreviated hr) is a unit of time conventionally reckoned as of a day and scientifically reckoned between 3,599 and 3,601 seconds, depending on the speed of Earth's rotation. There are 60 minutes in an hour, and 24 ...
s) is most commonly used in chemistry because it can be produced on a larger scale.
Mendelevium was discovered by bombarding einsteinium with alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be prod ...
s in 1955, the method still used to produce it today. It was named after Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
, father of the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
of the chemical elements. Using available microgram quantities of einsteinium-253, over a million mendelevium atoms may be made each hour. The chemistry of mendelevium is typical for the late actinides, with a preponderance of the +3 oxidation state but also an accessible +2 oxidation state. All known isotopes of mendelevium have short half-lives; there are currently no uses for it outside basic scientific research
The scientific method is an empirical method for acquiring knowledge that has characterized the development of science since at least the 17th century (with notable practitioners in previous centuries; see the article history of scientific m ...
, and only small amounts are produced.
Discovery
 Mendelevium was the ninth transuranic element to be synthesized. It was first synthesized by Albert Ghiorso, Glenn T. Seaborg,
Mendelevium was the ninth transuranic element to be synthesized. It was first synthesized by Albert Ghiorso, Glenn T. Seaborg, Gregory Robert Choppin
Gregory Robert Choppin (November 9, 1927, Texas, United States – October 21, 2015, Tallahassee, Florida) was an American nuclear chemist and co-discoverer of the element mendelevium, atomic number 101. Others in the discovery group were Albert G ...
, Bernard G. Harvey, and team leader Stanley G. Thompson in early 1955 at the University of California, Berkeley. The team produced 256Md (half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of 77 minutes) when they bombarded an 253 Es target consisting of only a billion (109) einsteinium atoms with alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be prod ...
s (helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic ta ...
nuclei) in the Berkeley Radiation Laboratory
Lawrence Berkeley National Laboratory (LBNL), commonly referred to as the Berkeley Lab, is a United States national laboratory that is owned by, and conducts scientific research on behalf of, the United States Department of Energy. Located in ...
's 60-inch cyclotron
A cyclotron is a type of particle accelerator invented by Ernest O. Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Jan ...
, thus increasing the target's atomic number by two. 256Md thus became the first isotope of any element to be synthesized one atom at a time. In total, seventeen mendelevium atoms were produced. This discovery was part of a program, begun in 1952, that irradiated plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
with neutrons to transmute it into heavier actinides. This method was necessary as the previous method used to synthesize transuranic elements, neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons ...
, could not work because of a lack of known beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
ing isotopes of fermium that would produce isotopes of the next element, mendelevium, and also due to the very short half-life to spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakd ...
of 258 Fm that thus constituted a hard limit to the success of the neutron capture process.
To predict if the production of mendelevium would be possible, the team made use of a rough calculation. The number of atoms that would be produced would be approximately equal to the product of the number of atoms of target material, the target's cross section, the ion beam intensity, and the time of bombardment; this last factor was related to the half-life of the product when bombarding for a time on the order of its half-life. This gave one atom per experiment. Thus under optimum conditions, the preparation of only one atom of element 101 per experiment could be expected. This calculation demonstrated that it was feasible to go ahead with the experiment. The target material, einsteinium-253, could be produced readily from irradiating plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
: one year of irradiation would give a billion atoms, and its three-week half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
meant that the element 101 experiments could be conducted in one week after the produced einsteinium was separated and purified to make the target. However, it was necessary to upgrade the cyclotron to obtain the needed intensity of 1014 alpha particles per second; Seaborg applied for the necessary funds.
While Seaborg applied for funding, Harvey worked on the einsteinium target, while Thomson and Choppin focused on methods for chemical isolation. Choppin suggested using α-hydroxyisobutyric acid to separate the mendelevium atoms from those of the lighter actinides. The actual synthesis was done by a recoil technique, introduced by Albert Ghiorso. In this technique, the einsteinium was placed on the opposite side of the target from the beam, so that the recoiling mendelevium atoms would get enough momentum
In Newtonian mechanics, momentum (more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. If is an object's mass ...
to leave the target and be caught on a catcher foil made of gold. This recoil target was made by an electroplating technique, developed by Alfred Chetham-Strode. This technique gave a very high yield, which was absolutely necessary when working with such a rare and valuable product as the einsteinium target material. The recoil target consisted of 109 atoms of 253Es which were deposited electrolytically on a thin gold foil. It was bombarded by 41 MeV alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be prod ...
s in the Berkeley cyclotron with a very high beam density of 6×1013 particles per second over an area of 0.05 cm2. The target was cooled by water or liquid helium, and the foil could be replaced.
Initial experiments were carried out in September 1954. No alpha decay was seen from mendelevium atoms; thus, Ghiorso suggested that the mendelevium had all decayed by electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. ...
to fermium and that the experiment should be repeated to search instead for spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakd ...
events. The repetition of the experiment happened in February 1955.
 On the day of discovery, 19 February, alpha irradiation of the einsteinium target occurred in three three-hour sessions. The cyclotron was in the
On the day of discovery, 19 February, alpha irradiation of the einsteinium target occurred in three three-hour sessions. The cyclotron was in the University of California
The University of California (UC) is a public land-grant research university system in the U.S. state of California. The system is composed of the campuses at Berkeley, Davis, University of California, Irvine, Irvine, University of Califor ...
campus, while the Radiation Laboratory was on the next hill. To deal with this situation, a complex procedure was used: Ghiorso took the catcher foils (there were three targets and three foils) from the cyclotron to Harvey, who would use aqua regia to dissolve it and pass it through an anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
-exchange resin
In polymer chemistry and materials science, resin is a solid or highly viscous substance of plant or synthetic origin that is typically convertible into polymers. Resins are usually mixtures of organic compounds. This article focuses on nat ...
column to separate out the transuranium elements from the gold and other products. The resultant drops entered a test tube, which Choppin and Ghiorso took in a car to get to the Radiation Laboratory as soon as possible. There Thompson and Choppin used a cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
-exchange resin column and the α-hydroxyisobutyric acid. The solution drops were collected on platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
disks and dried under heat lamps. The three disks were expected to contain respectively the fermium, no new elements, and the mendelevium. Finally, they were placed in their own counters, which were connected to recorders such that spontaneous fission events would be recorded as huge deflections in a graph showing the number and time of the decays. There thus was no direct detection, but by observation of spontaneous fission events arising from its electron-capture daughter 256Fm. The first one was identified with a "hooray" followed by a "double hooray" and a "triple hooray". The fourth one eventually officially proved the chemical identification of the 101st element, mendelevium. In total, five decays were reported up until 4 a.m. Seaborg was notified and the team left to sleep. Additional analysis and further experimentation showed the produced mendelevium isotope to have mass 256 and to decay by electron capture to fermium-256 with a half-life of 1.5 h.
Being the first of the second hundred of the chemical elements, it was decided that the element would be named "mendelevium" after the Russian chemist Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
, father of the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
. Because this discovery came during the Cold War
The Cold War is a term commonly used to refer to a period of geopolitical tension between the United States and the Soviet Union and their respective allies, the Western Bloc and the Eastern Bloc. The term '' cold war'' is used because t ...
, Seaborg had to request permission of the government of the United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country Continental United States, primarily located in North America. It consists of 50 U.S. state, states, a Washington, D.C., ...
to propose that the element be named for a Russian, but it was granted. The name "mendelevium" was accepted by the International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
(IUPAC) in 1955 with symbol "Mv", which was changed to "Md" in the next IUPAC General Assembly (Paris, 1957).
Characteristics
Physical
 In the
In the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, mendelevium is located to the right of the actinide fermium, to the left of the actinide nobelium, and below the lanthanide thulium. Mendelevium metal has not yet been prepared in bulk quantities, and bulk preparation is currently impossible.Silva, pp. 1634–5 Nevertheless, a number of predictions and some preliminary experimental results have been done regarding its properties.
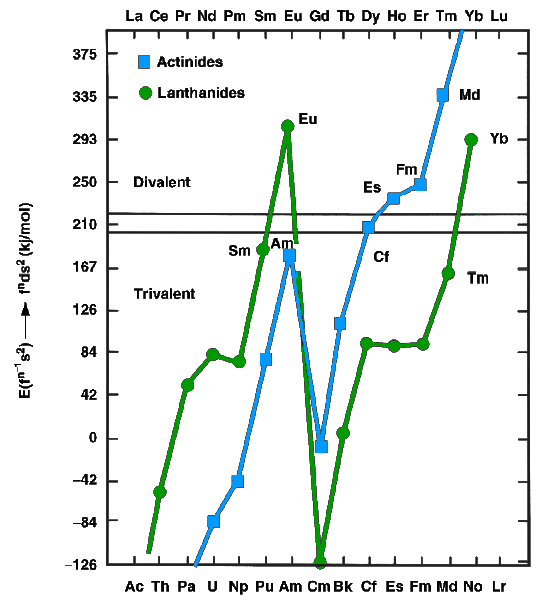
The lanthanides and actinides, in the metallic state, can exist as either divalent (such as europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lan ...
and ytterbium) or trivalent (most other lanthanides) metals. The former have f''n''s2 configurations, whereas the latter have f''n''−1d1s2 configurations. In 1975, Johansson and Rosengren examined the measured and predicted values for the cohesive energies ( enthalpies of crystallization) of the metallic lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yt ...
s and actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
s, both as divalent and trivalent metals.Silva, pp. 1626–8 The conclusion was that the increased binding energy of the nf126d17s2 configuration over the nf137s2 configuration for mendelevium was not enough to compensate for the energy needed to promote one 5f electron to 6d, as is true also for the very late actinides: thus einsteinium, fermium, mendelevium, and nobelium were expected to be divalent metals. The increasing predominance of the divalent state well before the actinide series concludes is attributed to the relativistic stabilization of the 5f electrons, which increases with increasing atomic number. Thermochromatographic studies with trace quantities of mendelevium by Zvara and Hübener from 1976 to 1982 confirmed this prediction. In 1990, Haire and Gibson estimated mendelevium metal to have an enthalpy of sublimation between 134 and 142 kJ/mol. Divalent mendelevium metal should have a metallic radius of around . Like the other divalent late actinides (except the once again trivalent lawrencium), metallic mendelevium should assume a face-centered cubic crystal structure. Mendelevium's melting point has been estimated at 827 °C, the same value as that predicted for the neighboring element nobelium. Its density is predicted to be around .
Chemical
The chemistry of mendelevium is mostly known only in solution, in which it can take on the +3 or +2oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s. The +1 state has also been reported, but has not yet been confirmed.Silva, pp. 1635–6
Before mendelevium's discovery, Seaborg and Katz predicted that it should be predominantly trivalent in aqueous solution and hence should behave similarly to other tripositive lanthanides and actinides. After the synthesis of mendelevium in 1955, these predictions were confirmed, first in the observation at its discovery that it eluted just after fermium in the trivalent actinide elution sequence from a cation-exchange column of resin, and later the 1967 observation that mendelevium could form insoluble hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. ...
s and fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts ty ...
s that coprecipitated with trivalent lanthanide salts. Cation-exchange and solvent extraction studies led to the conclusion that mendelevium was a trivalent actinide with an ionic radius somewhat smaller than that of the previous actinide, fermium. Mendelevium can form coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as '' ligands'' or complexing agents. M ...
es with 1,2-cyclohexanedinitrilotetraacetic acid (DCTA).
In reducing conditions, mendelevium(III) can be easily reduced to mendelevium(II), which is stable in aqueous solution. The standard reduction potential
Redox potential (also known as oxidation / reduction potential, ''ORP'', ''pe'', ''E_'', or E_) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respe ...
of the ''E''°(Md3+→Md2+) couple was variously estimated in 1967 as −0.10 V or −0.20 V: later 2013 experiments established the value as . In comparison, ''E''°(Md3+→Md0) should be around −1.74 V, and ''E''°(Md2+→Md0) should be around −2.5 V. Mendelevium(II)'s elution behavior has been compared with that of strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is e ...
(II) and europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lan ...
(II).
In 1973, mendelevium(I) was reported to have been produced by Russian scientists, who obtained it by reducing higher oxidation states of mendelevium with samarium
Samarium is a chemical element with symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. Compounds of samar ...
(II). It was found to be stable in neutral water–ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
solution and be homologous to caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
(I). However, later experiments found no evidence for mendelevium(I) and found that mendelevium behaved like divalent elements when reduced, not like the monovalent alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s. Nevertheless, the Russian team conducted further studies on the thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws ...
of cocrystallizing mendelevium with alkali metal chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride s ...
s, and concluded that mendelevium(I) had formed and could form mixed crystals with divalent elements, thus cocrystallizing with them. The status of the +1 oxidation state is still tentative.
The electrode potential ''E''°(Md4+→Md3+) was predicted in 1975 to be +5.4 V; 1967 experiments with the strong oxidizing agent sodium bismuthate were unable to oxidize mendelevium(III) to mendelevium(IV).
Atomic
A mendelevium atom has 101 electrons. They are expected to be arranged in the configuration nf137s2 (ground stateterm symbol In quantum mechanics, the term symbol is an abbreviated description of the (total) angular momentum quantum numbers in a multi-electron atom (however, even a single electron can be described by a term symbol). Each energy level of an atom with a giv ...
2F7/2), although experimental verification of this electron configuration had not yet been made as of 2006. The fifteen electrons in the 5f and 7s subshells are valence electrons.Silva, pp. 1633–4 In forming compounds, three valence electrons may be lost, leaving behind a nf12 core: this conforms to the trend set by the other actinides with their nnbsp;5f''n'' electron configurations in the tripositive state. The first ionization potential of mendelevium was measured to be at most (6.58 ± 0.07) eV in 1974, based on the assumption that the 7s electrons would ionize before the 5f ones; this value has since not yet been refined further due to mendelevium's scarcity and high radioactivity. The ionic radius of hexacoordinate Md3+ had been preliminarily estimated in 1978 to be around 91.2 pm; 1988 calculations based on the logarithmic trend between distribution coefficients and ionic radius produced a value of 89.6 pm, as well as an enthalpy of hydration of . Md2+ should have an ionic radius of 115 pm and hydration enthalpy −1413 kJ/mol; Md+ should have ionic radius 117 pm.
Isotopes
Seventeen isotopes of mendelevium are known, with mass numbers from 244 to 260; all are radioactive.Silva, pp. 1630–1 Additionally, five nuclear isomers are known: 245mMd, 247mMd, 249mMd, 254mMd, and 258mMd. Of these, the longest-lived isotope is 258Md with a half-life of 51.5 days, and the longest-lived isomer is 258mMd with a half-life of 58.0 minutes. Nevertheless, the shorter-lived 256Md (half-life 1.17 hours) is more often used in chemical experimentation because it can be produced in larger quantities fromalpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be prod ...
irradiation of einsteinium. After 258Md, the next most stable mendelevium isotopes are 260Md with a half-life of 31.8 days, 257Md with a half-life of 5.52 hours, 259Md with a half-life of 1.60 hours, and 256Md with a half-life of 1.17 hours. All of the remaining mendelevium isotopes have half-lives that are less than an hour, and the majority of these have half-lives that are less than 5 minutes.
The half-lives of mendelevium isotopes mostly increase smoothly from 244Md onwards, reaching a maximum at 258Md. Experiments and predictions suggest that the half-lives will then decrease, apart from 260Md with a half-life of 31.8 days, as spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakd ...
becomes the dominant decay mode due to the mutual repulsion of the protons posing a limit to the island of relative stability of long-lived nuclei in the actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
series.
Mendelevium-256, the chemically most important isotope of mendelevium, decays through electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. ...
90% of the time and alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
10% of the time. It is most easily detected through the spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakd ...
of its electron capture daughter fermium-256, but in the presence of other nuclides that undergo spontaneous fission, alpha decays at the characteristic energies for mendelevium-256 (7.205 and 7.139 MeV) can provide more useful identification.
Production and isolation
The lightest isotopes (244Md to 247Md) are mostly produced through bombardment ofbismuth
Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs ...
targets with heavy argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice a ...
ions, while slightly heavier ones (248Md to 253Md) are produced by bombarding plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
and americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was n ...
targets with ions of carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
and nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
. The most important and most stable isotopes are in the range from 254Md to 258Md and are produced through bombardment of einsteinium with alpha particles: einsteinium-253, -254, and -255 can all be used. 259Md is produced as a daughter
A daughter is a female offspring; a girl or a woman in relation to her parents. Daughterhood is the state of being someone's daughter. The male counterpart is a son. Analogously the name is used in several areas to show relations between groups ...
of 259 No, and 260Md can be produced in a transfer reaction between einsteinium-254 and oxygen-18. Typically, the most commonly used isotope 256Md is produced by bombarding either einsteinium-253 or -254 with alpha particles: einsteinium-254 is preferred when available because it has a longer half-life and therefore can be used as a target for longer. Using available microgram quantities of einsteinium, femtogram
To help compare different orders of magnitude, the following lists describe various mass levels between 10−59 kg and 1052 kg. The least massive thing listed here is a graviton, and the most massive thing is the observable universe. ...
quantities of mendelevium-256 may be produced.
The recoil momentum
In Newtonian mechanics, momentum (more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. If is an object's mass ...
of the produced mendelevium-256 atoms is used to bring them physically far away from the einsteinium target from which they are produced, bringing them onto a thin foil of metal (usually beryllium
Beryllium is a chemical element with the symbol Be and atomic number 4. It is a steel-gray, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with other elements to for ...
, aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
, platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
, or gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile ...
) just behind the target in a vacuum.Silva, pp. 1631–3 This eliminates the need for immediate chemical separation, which is both costly and prevents reusing of the expensive einsteinium target. The mendelevium atoms are then trapped in a gas atmosphere (frequently helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic ta ...
), and a gas jet from a small opening in the reaction chamber carries the mendelevium along. Using a long capillary tube, and including potassium chloride
Potassium chloride (KCl, or potassium salt) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt ...
aerosols in the helium gas, the mendelevium atoms can be transported over tens of meters to be chemically analyzed and have their quantity determined. The mendelevium can then be separated from the foil material and other fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
s by applying acid to the foil and then coprecipitating the mendelevium with lanthanum fluoride
Lanthanum trifluoride is a refractory ionic compound of lanthanum and fluorine.
The LaF3 structure
Bonding is ionic with lanthanum highly coordinated. The cation sits at the center of a trigonal prism. Nine fluorine atoms are close: three at ...
, then using a cation-exchange resin column with a 10% ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
solution saturated with hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dige ...
, acting as an eluant. However, if the foil is made of gold and thin enough, it is enough to simply dissolve the gold in aqua regia before separating the trivalent actinides from the gold using anion-exchange chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system ( ...
, the eluant being 6 M hydrochloric acid.
Mendelevium can finally be separated from the other trivalent actinides using selective elution from a cation-exchange resin column, the eluant being ammonia α-HIB. Using the gas-jet method often renders the first two steps unnecessary. The above procedure is the most commonly used one for the separation of transeinsteinium elements.
Another possible way to separate the trivalent actinides is via solvent extraction chromatography using bis-(2-ethylhexyl) phosphoric acid (abbreviated as HDEHP) as the stationary organic phase and nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
as the mobile aqueous phase. The actinide elution sequence is reversed from that of the cation-exchange resin column, so that the heavier actinides elute later. The mendelevium separated by this method has the advantage of being free of organic complexing agent compared to the resin column; the disadvantage is that mendelevium then elutes very late in the elution sequence, after fermium.
Another method to isolate mendelevium exploits the distinct elution properties of Md2+ from those of Es3+ and Fm3+. The initial steps are the same as above, and employs HDEHP for extraction chromatography, but coprecipitates the mendelevium with terbium fluoride instead of lanthanum fluoride. Then, 50 mg of chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hard ...
is added to the mendelevium to reduce it to the +2 state in 0.1 M hydrochloric acid with zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
or mercury. The solvent extraction then proceeds, and while the trivalent and tetravalent lanthanides and actinides remain on the column, mendelevium(II) does not and stays in the hydrochloric acid. It is then reoxidized to the +3 state using hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3 ...
and then isolated by selective elution with 2 M hydrochloric acid (to remove impurities, including chromium) and finally 6 M hydrochloric acid (to remove the mendelevium). It is also possible to use a column of cationite and zinc amalgam, using 1 M hydrochloric acid as an eluant, reducing Md(III) to Md(II) where it behaves like the alkaline earth metals. Thermochromatographic chemical isolation could be achieved using the volatile mendelevium hexafluoroacetylacetonate: the analogous fermium compound is also known and is also volatile.
Toxicity
Though few people come in contact with mendelevium, the International Commission on Radiological Protection has set annual exposure limits for the most stable isotope. For mendelevium-258, the ingestion limit was set at 9×105 becquerels (1 Bq = 1 decay per second). Given the half-life of this isotope, this is only 2.48 ng (nanograms). The inhalation limit is at 6000 Bq or 16.5 pg (picogram).Notes
References
Bibliography
*Further reading
* Hoffman, D.C., Ghiorso, A., Seaborg, G. T. The transuranium people: the inside story, (2000), 201–229 * Morss, L. R., Edelstein, N. M., Fuger, J., The chemistry of the actinide and transactinide element, 3, (2006), 1630–1636 * ''A Guide to the Elements – Revised Edition'', Albert Stwertka, (Oxford University Press; 1998)External links
Los Alamos National Laboratory – Mendelevium
at '' The Periodic Table of Videos'' (University of Nottingham)
Environmental Chemistry – Md info
{{Authority control Chemical elements Chemical elements with face-centered cubic structure Actinides Synthetic elements