magnesium-23 on:
[Wikipedia]
[Google]
[Amazon]
Magnesium isotopes data from ''The Berkeley Laboratory Isotopes Project's''
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
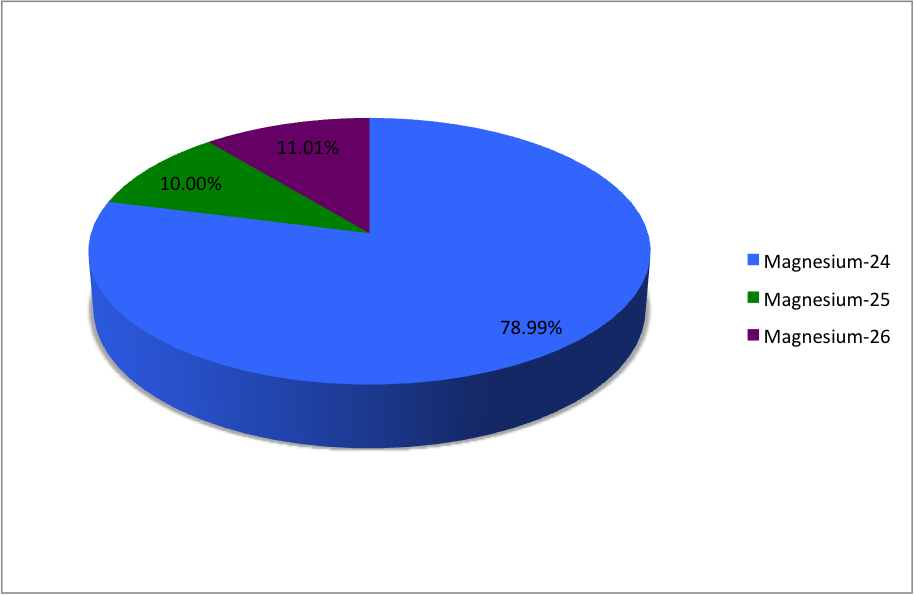
(12Mg) naturally occurs in three stable isotopes: , , and . There are 19 radioisotopes that have been discovered, ranging from to . The longest-lived radioisotope is with a half-life of . The lighter isotopes mostly decay to isotopes of sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
while the heavier isotopes decay to isotopes of aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
. The shortest-lived is proton-unbound with a half-life of , though the half-life of similarly unbound has not been measured.

List of isotopes
, - , , style="text-align:right" , 12 , style="text-align:right" , 6 , , , 2p , , 0+ , , , - , , style="text-align:right" , 12 , style="text-align:right" , 7 , , , 2p , , 1/2−# , , , - , rowspan=2, , rowspan=2 style="text-align:right" , 12 , rowspan=2 style="text-align:right" , 8 , rowspan=2, , rowspan=2, , β+ () , , rowspan=2, 0+ , rowspan=2, , rowspan=2, , - , β+p () , , - , rowspan=4, , rowspan=4 style="text-align:right" , 12 , rowspan=4 style="text-align:right" , 9 , rowspan=4, , rowspan=4, , β+ () , , rowspan=4, 5/2+ , rowspan=4, , rowspan=4, , - , β+p () , , - , β+α () , , - , β+pα () , , - , , style="text-align:right" , 12 , style="text-align:right" , 10 , , , β+ , , 0+ , , , - , , style="text-align:right" , 12 , style="text-align:right" , 11 , , , β+ , , 3/2+ , , , - , , style="text-align:right" , 12 , style="text-align:right" , 12 , , colspan=3 align=center, Stable , 0+ , colspan=2 align=center, , - , , style="text-align:right" , 12 , style="text-align:right" , 13 , , colspan=3 align=center, Stable , 5/2+ , colspan=2 align=center, , - , Used inradiodating
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to date materials such as rocks or carbon, in which trace radioactive impurities were selectively incorporated when they were formed. The method compares ...
events early in the Solar System's history
, style="text-align:right" , 12
, style="text-align:right" , 14
,
, colspan=3 align=center, Stable
, 0+
, colspan=2 align=center, , -
,
, style="text-align:right" , 12
, style="text-align:right" , 15
,
,
, β−
,
, 1/2+
,
,
, -
,
, style="text-align:right" , 12
, style="text-align:right" , 16
,
,
, β−
,
, 0+
,
,
, -
,
, style="text-align:right" , 12
, style="text-align:right" , 17
,
,
, β−
,
, 3/2+
,
,
, -
, rowspan=2,
, rowspan=2 style="text-align:right" , 12
, rowspan=2 style="text-align:right" , 18
, rowspan=2,
, rowspan=2,
, β− (> )
,
, rowspan=2, 0+
, rowspan=2,
, rowspan=2,
, -
, β−n (< )
,
, -
, rowspan=2,
, rowspan=2 style="text-align:right" , 12
, rowspan=2 style="text-align:right" , 19
, rowspan=2,
, rowspan=2,
, β− ()
,
, rowspan=2, 1/2+
, rowspan=2,
, rowspan=2,
, -
, β−n ()
,
, -
, rowspan=2,
, rowspan=2 style="text-align:right" , 12
, rowspan=2 style="text-align:right" , 20
, rowspan=2,
, rowspan=2,
, β− ()
,
, rowspan=2, 0+
, rowspan=2,
, rowspan=2,
, -
, β−n ()
,
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 21
, rowspan=3,
, rowspan=3,
, β− ()
,
, rowspan=3, 3/2−
, rowspan=3,
, rowspan=3,
, -
, β−n ()
,
, -
, β−2n ?Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide.
, ?
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 22
, rowspan=3,
, rowspan=3,
, β− (> )
,
, rowspan=3, 0+
, rowspan=3,
, rowspan=3,
, -
, β−n ()
,
, -
, β−2n (< )
,
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 23
, rowspan=3,
, rowspan=3,
, β−n ()
,
, rowspan=3, (3/2−, 5/2−)
, rowspan=3,
, rowspan=3,
, -
, β− ()
,
, -
, β−2n ?
, ?
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 24
, rowspan=3,
, rowspan=3,
, β− ()
,
, rowspan=3, 0+
, rowspan=3,
, rowspan=3,
, -
, β−n ()
,
, -
, β−2n ?
, ?
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 25
, rowspan=3,
, rowspan=3,
, β− ?
, ?
, rowspan=3, (3/2−)
, rowspan=3,
, rowspan=3,
, -
, β−n ?
, ?
, -
, β−2n ?
, ?
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 26
, rowspan=3, #
, rowspan=3, 2# ms , β− (100# %)
, #
, rowspan=3, 0+
, rowspan=3,
, rowspan=3,
, -
, β−n ?
, ?
, -
, β−2n ?
, ?
, -
, rowspan=2, ?This isotope has not yet been definitively observed; given data is inferred or estimated from periodic trends.
, rowspan=2 style="text-align:right" , 12
, rowspan=2 style="text-align:right" , 27
, rowspan=2, #
, rowspan=2, <
, n ?
, ?
, rowspan=2, 7/2−#
, rowspan=2,
, rowspan=2,
, -
, β− ?
, ?
, -
, rowspan=3,
, rowspan=3 style="text-align:right" , 12
, rowspan=3 style="text-align:right" , 28
, rowspan=3, #
, rowspan=3, 1# ms , β− ?
, ?
, rowspan=3, 0+
, rowspan=3,
, rowspan=3,
, -
, β−n ?
, ?
, -
, β−2n ?
, ?
, -
, rowspan=2, ?
, rowspan=2 style="text-align:right" , 12
, rowspan=2 style="text-align:right" , 29
, rowspan=2, #
, rowspan=2,
, β− ?
, ?
, rowspan=2, 3/2−#
, rowspan=2,
, rowspan=2,
, -
, β−n ?
, ?
External links
Magnesium isotopes data from ''The Berkeley Laboratory Isotopes Project's''
References
{{Navbox element isotopes MagnesiumMagnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...