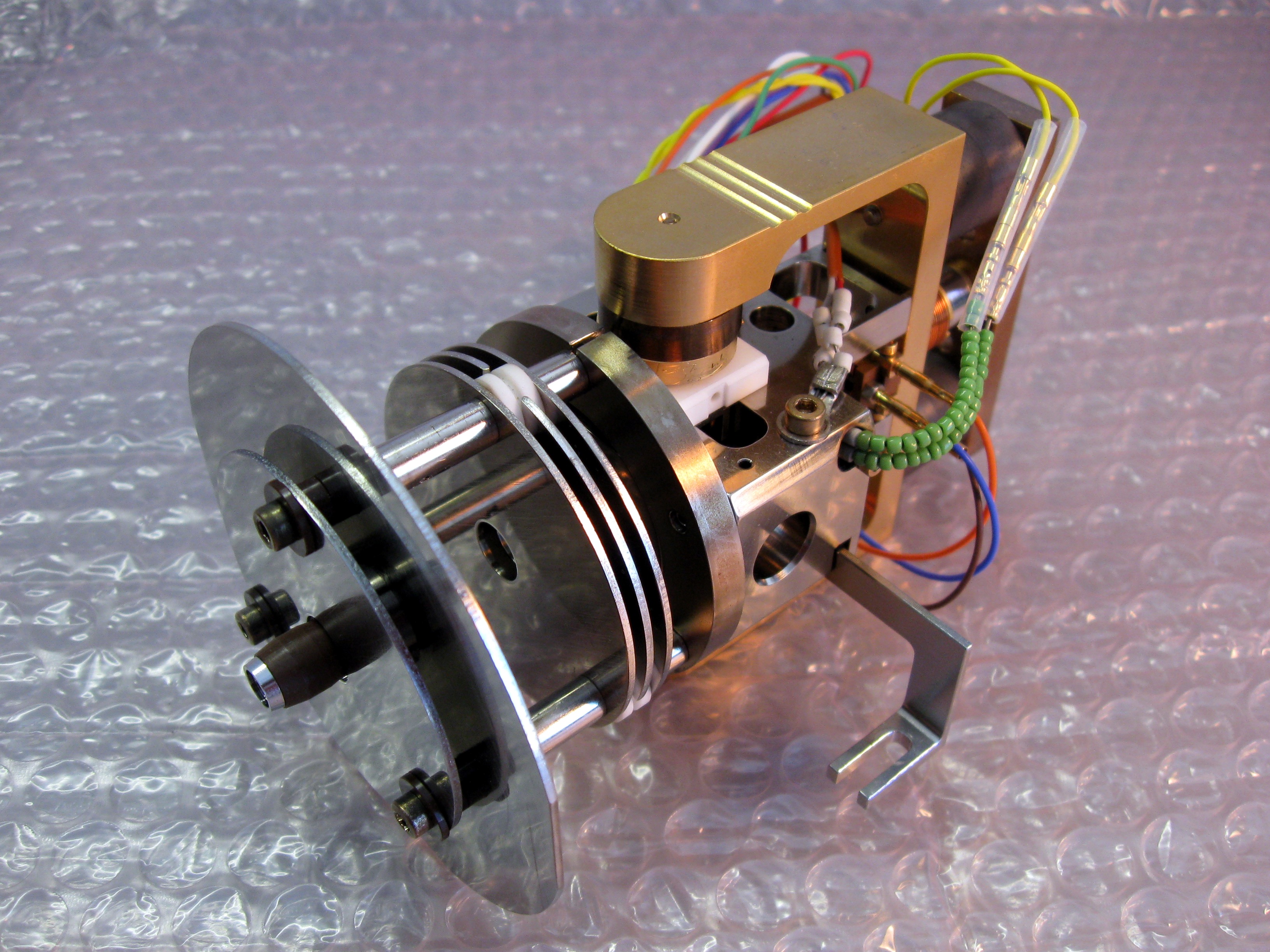
An ion source is a device that creates atomic and molecular
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conv ...
s.
Ion sources are used to form ions for
mass spectrometers,
optical emission spectrometers,
particle accelerator
A particle accelerator is a machine that uses electromagnetic fields to propel charged particles to very high speeds and energies, and to contain them in well-defined beams.
Large accelerators are used for fundamental research in particle ...
s,
ion implanters and
ion engines
An ion thruster, ion drive, or ion engine is a form of electric propulsion used for spacecraft propulsion. It creates thrust by accelerating ions using electricity.
An ion thruster ionizes a neutral gas by extracting some electrons out of ...
.
Electron ionization
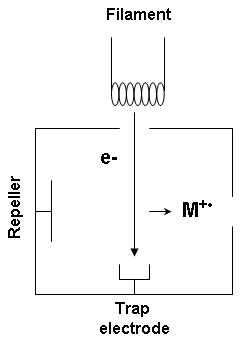
Electron ionization
Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of t ...
is widely used in mass spectrometry, particularly for
organic molecules. The
gas phase
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetizat ...
reaction producing electron ionization is
:
M + e^- -> M^ + 2e^-
where M is the atom or molecule being ionized,
e^- is the electron, and
M^ is the resulting ion.
The electrons may be created by an
arc discharge
An electric arc, or arc discharge, is an electrical breakdown of a gas that produces a prolonged electrical discharge. The current through a normally nonconductive medium such as air produces a plasma; the plasma may produce visible light. A ...
between a
cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
and an
anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic ...
.
An electron beam ion source (EBIS) is used in
atomic physics to produce highly charged
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conv ...
s by bombarding
atoms with a powerful
electron beam
Cathode rays or electron beam (e-beam) are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to ele ...
.
Its principle of operation is shared by the
electron beam ion trap
Electron beam ion trap (EBIT) is an electromagnetic bottle that produces and confines highly charged ions. An EBIT uses an electron beam focused with a powerful magnetic field to ionize atoms to high charge states by successive electron impact.
...
.
Electron capture ionization
Electron capture ionization (ECI) is the ionization of a gas phase
atom or
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
by attachment of an
electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kno ...
to create an ion of the form A
−•. The reaction is
:
A + e^- -> A^-
where the M over the arrow denotes that to conserve
energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat ...
and
momentum
In Newtonian mechanics, momentum (more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. If is an object's mass and ...
a third body is required (the
molecularity
In chemistry, molecularity is the number of molecules that come together to react in an elementary (single-step) reactionAtkins, P.; de Paula, J. Physical Chemistry. Oxford University Press, 2014 and is equal to the sum of stoichiometric coeffic ...
of the reaction is three).
Electron capture can be used in conjunction with
chemical ionization
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules (ofte ...
.
An
electron capture detector
An electron capture detector (ECD) is a device for detecting atoms and molecules in a gas through the attachment of electrons via electron capture ionization. The device was invented in 1957 by James Lovelock and is used in gas chromatography to d ...
is used in some
gas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, ...
systems.
Chemical ionization
Chemical ionization
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules (ofte ...
(CI) is a lower energy process than
electron ionization
Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of t ...
because it involves ion/molecule reactions rather than electron removal. The lower energy yields less
fragmentation, and usually a simpler
spectrum
A spectrum (plural ''spectra'' or ''spectrums'') is a condition that is not limited to a specific set of values but can vary, without gaps, across a continuum. The word was first used scientifically in optics to describe the rainbow of colors ...
. A typical CI spectrum has an easily identifiable molecular ion.
In a CI experiment, ions are produced through the collision of the analyte with ions of a reagent gas in the ion source. Some common reagent gases include:
methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on ...
,
ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
, and
isobutane
Isobutane, also known as ''i''-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH3)3. It is an isomer of butane. Isobutane is a colourless, odourless gas.
It is the simplest alkane with a tertiary carbon ...
. Inside the ion source, the reagent gas is present in large excess compared to the analyte. Electrons entering the source will preferentially ionize the reagent gas. The resultant collisions with other reagent gas molecules will create an ionization
plasma. Positive and negative ions of the analyte are formed by reactions with this plasma. For example,
protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, i ...
occurs by
:
CH4 + e^- -> CH4+ + 2e^- (primary ion formation),
:
CH4 + CH4+ -> CH5+ + CH3 (reagent ion formation),
:
M + CH5+ -> CH4 + + H (product ion formation, e.g. protonation).
Charge exchange ionization
Charge-exchange ionization (also known as charge-transfer ionization) is a gas phase reaction between an
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conv ...
and an
atom or
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
in which the charge of the ion is transferred to the neutral species.
:
A+ + B -> A + B+
Chemi-ionization
Chemi-ionization is the formation of an
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conv ...
through the reaction of a gas phase
atom or
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
with an atom or molecule in an
excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers ...
.
Chemi-ionization can be represented by
:
G^\ast + M -> G + M^ + e^-
where G is the excited state species (indicated by the superscripted asterisk), and M is the species that is ionized by the loss of an
electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kno ...
to form the
radical
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
(indicated by the superscripted "plus-dot").
Associative ionization
Associative ionization is a gas phase reaction in which two atoms or molecules interact to form a single product ion. One or both of the interacting species may have excess
internal energy
The internal energy of a thermodynamic system is the total energy contained within it. It is the energy necessary to create or prepare the system in its given internal state, and includes the contributions of potential energy and internal kinet ...
.
For example,
:
A^\ast + B -> AB^ + e^-
where species A with excess internal energy (indicated by the asterisk) interacts with B to form the ion AB
+.
Penning ionization
Penning ionization is a form of chemi-ionization involving reactions between neutral atoms or molecules.
The process is named after the Dutch physicist
Frans Michel Penning who first reported it in 1927. Penning ionization involves a reaction between a gas-phase excited-state atom or molecule G
* and a target molecule M resulting in the formation of a radical molecular cation M
+., an electron e
−, and a neutral gas molecule G:
:
G^\ast + M -> G + M^ + e^-
Penning ionization occurs when the target molecule has an
ionization potential
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule ...
lower than the internal energy of the excited-state atom or molecule.
Associative Penning ionization can proceed via
:
G^\ast + M -> MG^ + e^-
Surface Penning ionization (also known as Auger deexcitation) refers to the interaction of the excited-state gas with a bulk surface S, resulting in the release of an electron according to
:
G^\ast + S -> G + S + e^-.
Ion attachment
Ion-attachment ionization is similar to
chemical ionization
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules (ofte ...
in which a cation is attached to the analyte molecule in a reactive collision:
:
M + X+ + A -> MX+ + A
Where M is the analyte molecule, X
+ is the cation and A is a non-reacting collision partner.
In a radioactive ion source, a small piece of radioactive material, for instance
63 Ni or
241 Am, is used to ionize a gas. This is used in ionization
smoke detector
A smoke detector is a device that senses smoke, typically as an indicator of fire. Smoke detectors are usually housed in plastic enclosures, typically shaped like a disk about in diameter and thick, but shape and size vary. Smoke can be detecte ...
s and
ion mobility spectrometers.
Gas-discharge ion sources

These ion sources use a
or
electric discharge
An electric discharge is the release and transmission of electricity in an applied electric field through a medium such as a gas (ie., an outgoing flow of electric current through a non-metal medium).American Geophysical Union, National Research ...
to create ions.
Inductively-coupled plasma
Ions can be created in an
inductively coupled plasma
An inductively coupled plasma (ICP) or transformer coupled plasma (TCP) is a type of plasma source in which the energy is supplied by electric currents which are produced by electromagnetic induction, that is, by time-varying magnetic fields.
...
, which is a
plasma source in which the
energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat ...
is supplied by
electrical current
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by ...
s which are produced by
electromagnetic induction
Electromagnetic or magnetic induction is the production of an electromotive force (emf) across an electrical conductor in a changing magnetic field.
Michael Faraday is generally credited with the discovery of induction in 1831, and James Clerk ...
, that is, by time-varying
magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to ...
s.
Microwave-induced plasma
Microwave induced plasma ion sources are capable of exciting electrodeless gas discharges to create ions for trace element mass spectrometry.
A microwave plasma is a type of
plasma, that has high frequency
electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visible) ligh ...
in the
GHz
The hertz (symbol: Hz) is the unit of frequency in the International System of Units (SI), equivalent to one event (or cycle) per second. The hertz is an SI derived unit whose expression in terms of SI base units is s−1, meaning that one ...
range. It is capable of exciting
electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials de ...
less
gas discharge Electric discharge in gases occurs when electric current flows through a gaseous medium due to ionization of the gas. Depending on several factors, the discharge may radiate visible light. The properties of electric discharges in gases are studied i ...
s. If applied in
surface-wave-sustained mode, they are especially well suited to generate large-area plasmas of high plasma density. If they are both in
surface-wave and
resonator mode, they can exhibit a high degree of spatial localization. This allows to spatially separate the location of plasma generations from the location of surface processing. Such a separation (together with an appropriate gas-flow scheme) may help reduce the negative effect, that particles released from a processed substrate may have on the
plasma chemistry
Gas phase ion chemistry is a field of science encompassed within both chemistry and physics. It is the science that studies ions and molecules in the gas phase, most often enabled by some form of mass spectrometry. By far the most important appli ...
of the
gas phase
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetizat ...
.
ECR ion source
The ECR ion source makes use of the
electron cyclotron resonance
Electron cyclotron resonance (ECR) is a phenomenon observed in plasma physics, condensed matter physics, and accelerator physics. It happens when the frequency of incident radiation coincides with the natural frequency of rotation of electrons in ...
to ionize a plasma. Microwaves are injected into a volume at the frequency corresponding to the electron cyclotron resonance, defined by the magnetic field applied to a region inside the volume. The volume contains a low pressure gas.
Glow discharge
Ions can be created in an electric
glow discharge
A glow discharge is a plasma formed by the passage of electric current through a gas. It is often created by applying a voltage between two electrodes in a glass tube containing a low-pressure gas. When the voltage exceeds a value called the s ...
. A glow discharge is a
plasma formed by the passage of
electric current
An electric current is a stream of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is measured as the net rate of flow of electric charge through a surface or into a control volume. The moving pa ...
through a low-pressure gas. It is created by applying a voltage between two metal
electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials de ...
s in an evacuated chamber containing gas. When the voltage exceeds a certain value, called the
striking voltage, the gas forms a plasma.
A
duoplasmatron is a type of glow discharge ion source that consists of a
cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
(
hot cathode
In vacuum tubes and gas-filled tubes, a hot cathode or thermionic cathode is a cathode electrode which is heated to make it emit electrons due to thermionic emission. This is in contrast to a cold cathode, which does not have a heating element. ...
or
cold cathode
A cold cathode is a cathode that is not electrically heated by a filament.A negatively charged electrode emits electrons or is the positively charged terminal. For more, see field emission. A cathode may be considered "cold" if it emits more e ...
) that produces a plasma that is used to ionize a gas.
Duoplasmatrons can produce positive or negative ions.
Duoplasmatrons are used for secondary ion mass spectrometry.,
ion beam etching, and high-energy physics.
Flowing afterglow
In a flowing
afterglow
An afterglow in meteorology consists of several atmospheric optical phenomena, with a general definition as a broad arch of whitish or pinkish sunlight in the twilight sky, consisting of the bright segment and the purple light. Purple light mainl ...
, ions are formed in a flow of inert gas, typically
helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. It ...
or
argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
.
Reagents are added downstream to create ion products and study reaction rates.
Flowing-afterglow mass spectrometry is used for trace gas analysis
for organic compounds.
Spark ionization
Electric
spark ionization
Spark ionization (also known as spark source ionization) is a method used to produce gas phase ions from a solid sample. The prepared solid sample is vaporized and partially ionized by an intermittent discharge or spark. This technique is primar ...
is used to produce gas phase
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conv ...
s from a solid sample. When incorporated with a mass spectrometer the complete instrument is referred to as a spark ionization mass spectrometer or as a spark source mass spectrometer (SSMS).
A closed drift ion source uses a radial magnetic field in an annular cavity in order to confine electrons for ionizing a gas. They are used for
ion implantation
Ion implantation is a low-temperature process by which ions of one element are accelerated into a solid target, thereby changing the physical, chemical, or electrical properties of the target. Ion implantation is used in semiconductor device fa ...
and for space propulsion (
Hall-effect thruster
In spacecraft propulsion, a Hall-effect thruster (HET) is a type of ion thruster in which the propellant is accelerated by an electric field. Hall-effect thrusters (based on the discovery by Edwin Hall) are sometimes referred to as Hall thrust ...
s).
Photoionization
Photoionization
Photoionization is the physical process in which an ion is formed from the interaction of a photon with an atom or molecule.
Cross section
Not every interaction between a photon and an atom, or molecule, will result in photoionization. The prob ...
is the ionization process in which an ion is formed from the interaction of a
photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless, so they always m ...
with an atom or molecule.
Multi-photon ionization
In multi-photon ionization (MPI), several photons of energy below the ionization threshold may actually combine their energies to ionize an atom.
Resonance-enhanced multiphoton ionization (REMPI) is a form of MPI in which one or more of the photons accesses a
bound-bound transition that is
resonant
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillat ...
in the atom or molecule being ionized.
Atmospheric pressure photoionization
Atmospheric pressure photoionization
Atmospheric pressure photoionization (APPI) is a soft ionization method used in mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' ma ...
(APPI) uses a source of photons, usually a vacuum UV (VUV) lamp, to ionize the analyte with single photon ionization process. Analogous to other atmospheric pressure ion sources, a spray of solvent is heated to relatively high temperatures (above 400 degrees Celsius) and sprayed with high flow rates of nitrogen for desolvation. The resulting
aerosol
An aerosol is a suspension of fine solid particles or liquid droplets in air or another gas. Aerosols can be natural or anthropogenic. Examples of natural aerosols are fog or mist, dust, forest exudates, and geyser steam. Examples of anthropo ...
is subjected to UV radiation to create ions.
Atmospheric-pressure laser ionization uses UV laser light sources to ionize the analyte via MPI.
Desorption ionization
Field desorption

Field desorption
Field desorption (FD) is a method of ion formation used in mass spectrometry (MS) in which a high-potential electric field is applied to an ''emitter'' with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whisk ...
refers to an ion source in which a high-potential electric field is applied to an emitter with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers" have formed. This results in a very high electric field which can result in ionization of gaseous molecules of the analyte. Mass spectra produced by FI have little or no fragmentation. They are dominated by molecular radical cations
M^ and less often, protonated molecules
H.
Particle bombardment
Fast atom bombardment
Particle bombardment with atoms is called
fast atom bombardment
Fast atom bombardment (FAB) is an ionization technique used in mass spectrometry in which a beam of high energy atoms strikes a surface to create ions. It was developed by Michael Barber at the University of Manchester in 1980. When a beam of high ...
(FAB) and bombardment with atomic or molecular ions is called
secondary ion mass spectrometry
Secondary-ion mass spectrometry (SIMS) is a technique used to analyze the composition of solid surfaces and thin films by sputtering the surface of the specimen with a focused primary ion beam and collecting and analyzing ejected secondary ions. ...
(SIMS).
Fission fragment ionization uses ionic or neutral atoms formed as a result of the
nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioa ...
of a suitable
nuclide
A nuclide (or nucleide, from nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by Truman ...
, for example the
Californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding cur ...
isotope
252Cf.
In FAB the analytes is mixed with a non-volatile chemical protection environment called a
matrix
Matrix most commonly refers to:
* ''The Matrix'' (franchise), an American media franchise
** ''The Matrix'', a 1999 science-fiction action film
** "The Matrix", a fictional setting, a virtual reality environment, within ''The Matrix'' (franchis ...
and is bombarded under vacuum with a high energy (4000 to 10,000
electron volts
In physics, an electronvolt (symbol eV, also written electron-volt and electron volt) is the measure of an amount of kinetic energy gained by a single electron accelerating from rest through an electric potential difference of one volt in vacuum. ...
) beam of atoms.
The atoms are typically from an inert gas such as
argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
or
xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
. Common matrices include
glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known ...
,
thioglycerol,
3-nitrobenzyl alcohol (3-NBA),
18-crown-6
18-Crown-6 is an organic compound with the formula 2H4Osub>6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ...
ether,
2-nitrophenyloctyl ether,
sulfolane
Sulfolane (also ''tetramethylene sulfone'', systematic name: 1λ6-thiolane-1,1-dione) is an organosulfur compound, formally a cyclic sulfone, with the formula (CH2)4SO2. It is a colorless liquid commonly used in the chemical industry as a solvent ...
,
diethanolamine
Diethanolamine, often abbreviated as DEA or DEOA, is an organic compound with the formula HN(CH2CH2OH)2. Pure diethanolamine is a white solid at room temperature, but its tendencies to absorb water and to supercool meaning that it is often encou ...
, and
triethanolamine
Triethanolamine, or TEA is a viscous organic compound that is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Approximately 150,000 tonnes were produced in 1999. It is a colourless compound although sampl ...
. This technique is similar to
secondary ion mass spectrometry
Secondary-ion mass spectrometry (SIMS) is a technique used to analyze the composition of solid surfaces and thin films by sputtering the surface of the specimen with a focused primary ion beam and collecting and analyzing ejected secondary ions. ...
and
plasma desorption mass spectrometry.
Secondary ionization
Secondary ion mass spectrometry (SIMS) is used to analyze the composition of solid surfaces and thin films by sputtering the surface of the specimen with a focused primary ion beam and collecting and analyzing ejected secondary ions. The mass/charge ratios of these secondary ions are measured with a mass spectrometer to determine the elemental, isotopic, or molecular composition of the surface to a depth of 1 to 2 nm.
In a
liquid metal ion source (LMIS), a metal (typically
gallium
Gallium is a chemical element with the symbol Ga and atomic number 31. Discovered by French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in group 13 of the periodic table and is similar to the other metals of the group (alumini ...
) is heated to the liquid state and provided at the end of a capillary or a needle. Then a
Taylor cone
A Taylor cone refers to the cone observed in electrospinning, electrospraying and hydrodynamic spray processes from which a jet of charged particles emanates above a threshold voltage. Aside from electrospray ionization in mass spectrometry, th ...
is formed under the application of a strong electric field. As the cone's tip get sharper, the electric field becomes stronger, until ions are produced by field evaporation. These ion sources are particularly used in
ion implantation
Ion implantation is a low-temperature process by which ions of one element are accelerated into a solid target, thereby changing the physical, chemical, or electrical properties of the target. Ion implantation is used in semiconductor device fa ...
or in
focused ion beam
Focused ion beam, also known as FIB, is a technique used particularly in the semiconductor industry, materials science and increasingly in the biological field for site-specific analysis, deposition, and ablation of materials. A FIB setup is a s ...
instruments.
Plasma desorption ionization

Plasma desorption ionization mass spectrometry (PDMS), also called fission fragment ionization, is a mass spectrometry technique in which ionization of material in a solid sample is accomplished by bombarding it with ionic or neutral atoms formed as a result of the
nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioa ...
of a suitable
nuclide
A nuclide (or nucleide, from nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was coined by Truman ...
, typically the
californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding cur ...
isotope
252Cf.
Laser desorption ionization

Matrix-assisted laser desorption/ionization
In mass spectrometry, matrix-assisted laser desorption/ionization (MALDI) is an ionization technique that uses a laser energy absorbing matrix to create ions from large molecules with minimal fragmentation. It has been applied to the analysis of ...
(MALDI) is a soft ionization technique. The sample is mixed with a matrix material. Upon receiving a laser pulse, the matrix absorbs the laser energy and it is thought that primarily the matrix is desorbed and ionized (by addition of a proton) by this event. The analyte molecules are also desorbed. The matrix is then thought to transfer proton to the analyte molecules (e.g., protein molecules), thus charging the analyte.
Surface-assisted laser desorption/ionization
Surface-assisted laser desorption/ionization (SALDI) is a
soft laser desorption technique used for analyzing
biomolecule
A biomolecule or biological molecule is a loosely used term for molecules present in organisms that are essential to one or more typically biological processes, such as cell division, morphogenesis, or development. Biomolecules include large ...
s by
mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
.
In its first embodiment, it used
graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on lar ...
matrix.
At present, laser desorption/ionization methods using other
inorganic
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistr ...
matrices, such as
nanomaterials
*
Nanomaterials describe, in principle, materials of which a single unit is sized (in at least one dimension) between 1 and 100 nm (the usual definition of nanoscale).
Nanomaterials research takes a materials science-based approach to nan ...
, are often regarded as SALDI variants. A related method named "ambient SALDI" - which is a combination of conventional SALDI with ambient mass spectrometry incorporating the
DART ion source In mass spectrometry, direct analysis in real time (DART) is an ion source that produces electronically or vibronically excited-state species from gases such as helium, argon, or nitrogen that ionize atmospheric molecules or dopant molecules. The ...
- has also been demonstrated.
Surface-enhanced laser desorption/ionization
Surface-enhanced laser desorption/ionization (SELDI) is a variant of MALDI that is used for the analysis of
protein mixture
In chemistry, a mixture is a material made up of two or more different chemical substances which are not chemically bonded. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the ...
s that uses a target modified to achieve biochemical
affinity with the analyte compound.
Desorption ionization on silicon
Desorption ionization on silicon (DIOS) refers to laser desorption/ionization of a sample deposited on a porous silicon surface.
Smalley source
A laser vaporization cluster source produces ions using a combination of laser desorption ionization and supersonic expansion.
The Smalley source (or Smalley cluster source) was developed by
Richard Smalley
Richard Errett Smalley (June 6, 1943 – October 28, 2005) was an American chemist who was the Gene and Norman Hackerman Professor of Chemistry, Physics, and Astronomy at Rice University. In 1996, along with Robert Curl, also a professor of ...
at
Rice University
William Marsh Rice University (Rice University) is a private research university in Houston, Texas. It is on a 300-acre campus near the Houston Museum District and adjacent to the Texas Medical Center. Rice is ranked among the top universitie ...
in the 1980s and was central to the discovery of
fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
s in 1985.
Aerosol ionization
In
aerosol mass spectrometry with time-of-flight analysis, micrometer sized solid aerosol particles extracted from the atmosphere are simultaneously desorbed and ionized by a precisely timed laser pulse as they pass through the center of a time-of-flight ion extractor.
Spray ionization

Spray ionization methods involve the formation of aerosol particles from a liquid
solution and the formation of bare ions after solvent evaporation.
Solvent-assisted ionization (SAI) is a method in which charged droplets are produced by introducing a solution containing analyte into a heated inlet tube of an atmospheric pressure ionization mass spectrometer. Just as in Electrospray Ionization (ESI), desolvation of the charged droplets produces multiply charged analyte ions. Volatile and nonvolatile compounds are analyzed by SAI, and high voltage is not required to achieve sensitivity comparable to ESI.
Application of a voltage to the solution entering the hot inlet through a zero dead volume fitting connected to fused silica tubing produces ESI-like mass spectra, but with higher sensitivity.
The inlet tube to the mass spectrometer becomes the ion source.
Matrix-Assisted Ionization
Matrix-Assisted Ionization
AIis similar to MALDI in sample preparation, but a laser is not required to convert analyte molecules included in a matrix compound into gas-phase ions. In MAI, analyte ions have charge states similar to electrospray ionization but obtained from a solid matrix rather than a solvent. No voltage or laser is required, but a laser can be used to obtain spatial resolution for imaging. Matrix-analyte samples are ionized in the vacuum of a mass spectrometer and can be inserted into the vacuum through an atmospheric pressure inlet. Less volatile matrices such as 2,5-dihydroxybenzoic acid require a hot inlet tube to produce analyte ions by MAI, but more volatile matrices such as 3-nitrobenzonitrile require no heat, voltage, or laser. Simply introducing the matrix:analyte sample to the inlet aperture of an atmospheric pressure ionization mass spectrometer produces abundant ions. Compounds at least as large as bovine serum albumin
6 kDacan be ionized with this method.
In this simple, low cost and easy to use ionization method, the inlet to the mass spectrometer can be considered the ion source.
Atmospheric-pressure chemical ionization
Atmospheric-pressure chemical ionization
Atmospheric pressure chemical ionization (APCI) is an ionization method used in mass spectrometry which utilizes gas-phase ion-molecule reactions at atmospheric pressure (105 Pa), commonly coupled with high-performance liquid chromatography (HPLC ...
is a form of
chemical ionization
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules (ofte ...
using a solvent spray at atmospheric pressure.
A spray of solvent is heated to relatively high temperatures (above 400 degrees Celsius), sprayed with high flow rates of nitrogen and the entire aerosol cloud is subjected to a
corona discharge
A corona discharge is an electrical discharge caused by the ionization of a fluid such as air surrounding a conductor carrying a high voltage. It represents a local region where the air (or other fluid) has undergone electrical breakdown a ...
that creates ions with the evaporated solvent acting as the chemical ionization reagent gas. APCI is not as "soft" (low fragmentation) an ionization technique as ESI.
Note that atmospheric pressure ionization (API) should not be used as a synonym for APCI.
Thermospray ionization
Thermospray ionization is a form of atmospheric pressure ionization in
mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
. It transfers ions from the liquid phase to the gas phase for analysis. It is particularly useful in
liquid chromatography-mass spectrometry
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, ...
.

Electrospray ionization
In
electrospray ionization
Electrospray ionization (ESI) is a technique used in mass spectrometry to produce ions using an electrospray in which a high voltage is applied to a liquid to create an aerosol. It is especially useful in producing ions from macromolecules becaus ...
, a
liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, an ...
is pushed through a very small, charged and usually
metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typica ...
,
capillary
A capillary is a small blood vessel from 5 to 10 micrometres (μm) in diameter. Capillaries are composed of only the tunica intima, consisting of a thin wall of simple squamous endothelial cells. They are the smallest blood vessels in the body: ...
. This liquid contains the substance to be studied, the
analyte
An analyte, component (in clinical chemistry), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The purest substances are referred to as analytes, such as 24 karat gold, NaCl, water, e ...
, dissolved in a large amount of
solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
, which is usually much more
volatile than the analyte. Volatile
acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s,
bases or
buffers are often added to this solution too. The analyte exists as an
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conv ...
in solution either in its anion or cation form. Because like
charges repel, the liquid pushes itself out of the capillary and forms an
aerosol
An aerosol is a suspension of fine solid particles or liquid droplets in air or another gas. Aerosols can be natural or anthropogenic. Examples of natural aerosols are fog or mist, dust, forest exudates, and geyser steam. Examples of anthropo ...
, a mist of small droplets about 10
μm across. The aerosol is at least partially produced by a process involving the formation of a
Taylor cone
A Taylor cone refers to the cone observed in electrospinning, electrospraying and hydrodynamic spray processes from which a jet of charged particles emanates above a threshold voltage. Aside from electrospray ionization in mass spectrometry, th ...
and a jet from the tip of this cone. An uncharged carrier gas such as
nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at sevent ...
is sometimes used to help
nebulize the liquid and to help
evaporate
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humid ...
the neutral solvent in the droplets. As the solvent evaporates, the analyte molecules are forced closer together, repel each other and break up the droplets. This process is called Coulombic fission because it is driven by repulsive
Coulombic force
Coulomb's inverse-square law, or simply Coulomb's law, is an experimental law of physics that quantifies the amount of force between two stationary, electrically charged particles. The electric force between charged bodies at rest is convention ...
s between charged molecules. The process repeats until the analyte is free of solvent and is a bare
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conv ...
. The ions observed are created by the addition of a
proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
(a hydrogen ion) and denoted
H, or of another
cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
such as
sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable is ...
ion,
+ Na, or the removal of a proton,
- H-. Multiply charged ions such as
2H2+ are often observed. For large
macromolecules
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The ...
, there can be many charge states, occurring with different frequencies; the charge can be as great as
+ 25H, for example.
Probe electrospray ionization
Probe electrospray ionization (PESI) is a modified version of electrospray, where the capillary for sample solution transferring is replaced by a sharp-tipped solid needle with periodical motion.
Contactless atmospheric pressure ionization
Contactless atmospheric pressure ionization is a technique used for analysis of liquid and solid samples by
mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used ...
.
Contactless API can be operated without an additional
electric power
Electric power is the rate at which electrical energy is transferred by an electric circuit. The SI unit of power is the watt, one joule per second. Standard prefixes apply to watts as with other SI units: thousands, millions and billions of ...
supply (supplying
voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to mo ...
to the source emitter), gas supply, or
syringe pump
A syringe driver, also known as a syringe pump, is a small infusion pump, used to gradually administer small amounts of fluid (with or without medication) to a patient or for use in chemical and biomedical research. Some syringe drivers can bot ...
. Thus, the technique provides a facile means for analyzing
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one elemen ...
s by mass spectrometry at
atmospheric pressure
Atmospheric pressure, also known as barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1013.25 millibars, ...
.
Sonic spray ionization
Sonic spray ionization is method for creating
ion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conv ...
s from a liquid solution, for example, a mixture of
methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a lig ...
and
water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
.
A
pneumatic
Pneumatics (from Greek ‘wind, breath’) is a branch of engineering that makes use of gas or pressurized air.
Pneumatic systems used in industry are commonly powered by compressed air or compressed inert gases. A centrally located and ele ...
nebulizer
In medicine, a nebulizer (American English) or nebuliser (British English) is a drug delivery device used to administer medication in the form of a mist inhaled into the lungs. Nebulizers are commonly used for the treatment of asthma, cystic fibr ...
is used to turn the solution into a
supersonic
Supersonic speed is the speed of an object that exceeds the speed of sound (Mach 1). For objects traveling in dry air of a temperature of 20 °C (68 °F) at sea level, this speed is approximately . Speeds greater than five times ...
spray of small droplets. Ions are formed when the solvent
evaporate
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humid ...
s and the statistically unbalanced charge distribution on the droplets leads to a net charge and complete desolvation results in the formation of ions. Sonic spray ionization is used to analyze small organic molecules and drugs and can analyze large molecules when an electric field is applied to the capillary to help increase the charge density and generate multiple charged ions of proteins.
Sonic spray ionization has been coupled with
high performance liquid chromatography
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pa ...
for the analysis of drugs.
Oligonucleotides have been studied with this method.
SSI has been used in a manner similar to desorption electrospray ionization
for
ambient ionization
Ambient ionization is a form of ionization in which ions are formed in an ion source outside the mass spectrometer without sample preparation or separation. Ions can be formed by extraction into charged electrospray droplets, thermally desorbed and ...
and has been coupled with
thin-layer chromatography
Thin-layer chromatography (TLC) is a chromatography technique used to separate non-volatile mixtures.
Thin-layer chromatography is performed on a sheet of an inert substrate such as glass, plastic, or aluminium foil, which is coated with a t ...
in this manner.
Ultrasonication-assisted spray ionization
Ultrasonication-assisted spray ionization (UASI) involves ionization through the application of
ultrasound
Ultrasound is sound waves with frequencies higher than the upper audible limit of human hearing. Ultrasound is not different from "normal" (audible) sound in its physical properties, except that humans cannot hear it. This limit varies ...
.
Thermal ionization
Thermal ionization
Thermal ionization, also known as surface ionization or contact ionization, is a physical process whereby the atoms are desorbed from a hot surface, and in the process are ionized.
Thermal ionization is used to make simple ion sources, for mass s ...
(also known as surface ionization, or contact ionization) involves spraying vaporized, neutral atoms onto a hot surface, from which the atoms re-evaporate in ionic form. To generate positive ions, the atomic species should have a low
ionization energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule ...
, and the surface should have a high
work function
In solid-state physics, the work function (sometimes spelt workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" m ...
. This technique is most suitable for
alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of ...
atoms (Li, Na, K, Rb, Cs) which have low ionization energies and are easily evaporated.
To generate negative ions, the atomic species should have a high
electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
Note that this is ...
, and the surface should have a low work function. This second approach is most suited for
halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
atoms Cl, Br, I, At.
Ambient ionization

In
ambient ionization
Ambient ionization is a form of ionization in which ions are formed in an ion source outside the mass spectrometer without sample preparation or separation. Ions can be formed by extraction into charged electrospray droplets, thermally desorbed and ...
, ions are formed outside the mass spectrometer without sample preparation or separation.
Ions can be formed by extraction into charged
electrospray
The name electrospray is used for an apparatus that employs electricity to disperse a liquid or for the fine aerosol resulting from this process. High voltage is applied to a liquid supplied through an emitter (usually a glass or metallic capilla ...
droplets, thermally desorbed and ionized by
chemical ionization
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules (ofte ...
, or laser
desorbed
Desorption is the physical process where a previously adsorbed substance is released from a surface. This happens when a molecule gains enough energy to overcome the activation barrier of the bounding energy that keeps it in the surface.
There ...
or
ablated and post-ionized before they enter the mass spectrometer.
Solid-liquid extraction based ambient ionization uses a charged spray to create a liquid film on the sample surface.
Molecules on the surface are extracted into the solvent. The action of the primary droplets hitting the surface produces secondary droplets that are the source of ions for the mass spectrometer. Desorption electrospray ionization (DESI) uses an
electrospray
The name electrospray is used for an apparatus that employs electricity to disperse a liquid or for the fine aerosol resulting from this process. High voltage is applied to a liquid supplied through an emitter (usually a glass or metallic capilla ...
source to create charged droplets that are directed at a solid sample a few millimeters to a few centimeters away. The charged droplets pick up the sample through interaction with the surface and then form highly charged ions that can be sampled into a mass spectrometer.
Plasma-based ambient ionization is based on an
electrical discharge
An electric discharge is the release and transmission of electricity in an applied electric field through a medium such as a gas (ie., an outgoing flow of electric current through a non-metal medium).American Geophysical Union, National Research C ...
in a flowing gas that produces metastable atoms and molecules and reactive ions. Heat is often used to assist in the desorption of volatile species from the sample. Ions are formed by
chemical ionization
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules (ofte ...
in the gas phase. A
direct analysis in real time source operates by exposing the sample to a dry gas stream (typically helium or nitrogen) that contains long-lived electronically or vibronically excited neutral
atoms
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
or
molecules
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
(or
"metastables").
Excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers ...
s are typically formed in the DART source by creating a
glow discharge
A glow discharge is a plasma formed by the passage of electric current through a gas. It is often created by applying a voltage between two electrodes in a glass tube containing a low-pressure gas. When the voltage exceeds a value called the s ...
in a chamber through which the gas flows. A similar method called atmospheric solids analysis probe
SAP
Sap is a fluid transported in xylem cells (vessel elements or tracheids) or phloem sieve tube elements of a plant. These cells transport water and nutrients throughout the plant.
Sap is distinct from latex, resin, or cell sap; it is a separ ...
uses the heated gas from ESI or APCI probes to vaporize sample placed on a melting point tube inserted into an ESI/APCI source.
Ionization is by APCI.
Laser-based ambient ionization is a two-step process in which a pulsed laser is used to desorb or ablate material from a sample and the plume of material interacts with an electrospray or plasma to create ions. Electrospray-assisted
laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The firs ...
desorption/ionization (ELDI) uses a 337 nm UV laser
or 3 μm infrared laser
to desorb material into an electrospray source.
Matrix-assisted laser desorption electrospray ionization Matrix-assisted laser desorption electrospray ionization (MALDESI) was first introduced in 2006 as a novel ambient ionization technique which combines the benefits of electrospray ionization (ESI) and matrix-assisted laser desorption/ionization ( ...
(MALDESI) is an atmospheric pressure ionization source for generation of multiply charged ions. An ultraviolet or infrared laser is directed onto a solid or liquid sample containing the analyte of interest and matrix desorbing neutral analyte molecules that are ionized by interaction with electrosprayed solvent droplets generating multiply charged ions.
Laser ablation electrospray ionization
Laser ablation electrospray ionization (LAESI) is an ambient ionization method for mass spectrometry that combines laser ablation from a mid-infrared (mid-IR) laser with a secondary electrospray ionization (ESI) process. The mid-IR laser is used t ...
(LAESI) is an
ambient ionization
Ambient ionization is a form of ionization in which ions are formed in an ion source outside the mass spectrometer without sample preparation or separation. Ions can be formed by extraction into charged electrospray droplets, thermally desorbed and ...
method for mass spectrometry that combines laser ablation from a mid-infrared (mid-IR) laser with a secondary
electrospray ionization
Electrospray ionization (ESI) is a technique used in mass spectrometry to produce ions using an electrospray in which a high voltage is applied to a liquid to create an aerosol. It is especially useful in producing ions from macromolecules becaus ...
(ESI) process.
Applications
Mass spectrometry
In a
mass spectrometer
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
a sample is ionized in an ion source and the resulting ions are separated by their mass-to-charge ratio. The ions are detected and the results are displayed as spectra of the relative abundance of detected ions as a function of the mass-to-charge ratio. The atoms or molecules in the sample can be identified by correlating known masses to the identified masses or through a characteristic fragmentation pattern.
Particle accelerators


In
particle accelerator
A particle accelerator is a machine that uses electromagnetic fields to propel charged particles to very high speeds and energies, and to contain them in well-defined beams.
Large accelerators are used for fundamental research in particle ...
s an ion source creates a
particle beam
A particle beam is a stream of charged or neutral particles. In particle accelerators, these particles can move with a velocity close to the speed of light. There is a difference between the creation and control of charged particle beams and n ...
at the beginning of the machine, the ''source''. The technology to create ion sources for particle accelerators depends strongly on the type of particle that needs to be generated:
electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kno ...
s,
proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s,
H− ion or a
Heavy ions
Heavy may refer to:
Measures
* Heavy (aeronautics), a term used by pilots and air traffic controllers to refer to aircraft capable of 300,000 lbs or more takeoff weight
* Heavy, a characterization of objects with substantial weight
* Heavy, ...
.
Electrons are generated with an
electron gun
An electron gun (also called electron emitter) is an electrical component in some vacuum tubes that produces a narrow, collimated electron beam that has a precise kinetic energy. The largest use is in cathode-ray tubes (CRTs), used in nearly ...
, of which there are many varieties.
Protons are generated with a
plasma-based device, like a
duoplasmatron or a
magnetron
The cavity magnetron is a high-power vacuum tube used in early radar systems and currently in microwave ovens and linear particle accelerators. It generates microwaves using the interaction of a stream of electrons with a magnetic field while ...
.
H− ions are generated with a
magnetron
The cavity magnetron is a high-power vacuum tube used in early radar systems and currently in microwave ovens and linear particle accelerators. It generates microwaves using the interaction of a stream of electrons with a magnetic field while ...
or a
Penning source. A magnetron consists of a central cylindrical cathode surrounded by an anode. The discharge voltage is typically greater than 150 V and the current drain is around 40 A. A
magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to ...
of about 0.2
tesla is parallel to the
cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
axis. Hydrogen gas is introduced by a pulsed gas valve.
Caesium
Caesium ( IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
is often used to lower the
work function
In solid-state physics, the work function (sometimes spelt workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" m ...
of the cathode, enhancing the amount of ions that are produced. Large caesiated
H− sources are also used for
plasma heating in nuclear fusion devices.
For a
Penning source, a strong magnetic field parallel to the electric field of the sheath guides electrons and ions on cyclotron spirals from cathode to cathode. Fast H-minus ions are generated at the cathodes as in the magnetron. They are slowed down due to the charge exchange reaction as they migrate to the plasma aperture. This makes for a beam of ions that is colder than the ions obtained from a magnetron.
Heavy ions
Heavy may refer to:
Measures
* Heavy (aeronautics), a term used by pilots and air traffic controllers to refer to aircraft capable of 300,000 lbs or more takeoff weight
* Heavy, a characterization of objects with substantial weight
* Heavy, ...
can be generated with an
electron cyclotron resonance
Electron cyclotron resonance (ECR) is a phenomenon observed in plasma physics, condensed matter physics, and accelerator physics. It happens when the frequency of incident radiation coincides with the natural frequency of rotation of electrons in ...
ion source. The use of electron cyclotron resonance (ECR) ion sources for the production of intense beams of highly charged ions has immensely grown over the last decade. ECR ion sources are used as injectors into linear accelerators, Van-de-Graaff generators or cyclotrons in nuclear and elementary particle physics. In atomic and surface physics ECR ion sources deliver intense beams of highly charged ions for collision experiments or for the investigation of surfaces. For the highest charge states, however,
Electron beam ion source
An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.
Electron ionization
Elect ...
s (EBIS) are needed. They can generate even bare ions of mid-heavy elements. The
Electron beam ion trap
Electron beam ion trap (EBIT) is an electromagnetic bottle that produces and confines highly charged ions. An EBIT uses an electron beam focused with a powerful magnetic field to ionize atoms to high charge states by successive electron impact.
...
(EBIT), based on the same principle, can produce up to bare uranium ions and can be used as an ion source as well.
Heavy ions
Heavy may refer to:
Measures
* Heavy (aeronautics), a term used by pilots and air traffic controllers to refer to aircraft capable of 300,000 lbs or more takeoff weight
* Heavy, a characterization of objects with substantial weight
* Heavy, ...
can also be generated with an
Ion Gun which typically uses the thermionic emission of electrons to ionize a substance in its gaseous state. Such instruments are typically used for surface analysis.
Gas flows through the ion source between the
anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic ...
and the
cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
. A positive
voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to mo ...
is applied to the anode. This voltage, combined with the high
magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to ...
between the tips of the internal and external cathodes allow a
plasma to start.
Ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
from the plasma are repelled by the anode
electric field
An electric field (sometimes E-field) is the physical field that surrounds electrically charged particles and exerts force on all other charged particles in the field, either attracting or repelling them. It also refers to the physical field f ...
. This creates an ion beam.
Surface modification
* Surface cleaning and pretreatment for large area deposition
*
Thin film
A thin film is a layer of material ranging from fractions of a nanometer (monolayer) to several micrometers in thickness. The controlled synthesis of materials as thin films (a process referred to as deposition) is a fundamental step in many a ...
deposition
* Deposition of thick
diamond-like carbon
Diamond-like carbon (DLC) is a class of amorphous carbon material that displays some of the typical properties of diamond. DLC is usually applied as coatings to other materials that could benefit from such properties.
DLC exists in seven diffe ...
(DLC) films
* Surface roughening of
polymers
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and ...
for improved
adhesion
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another ( cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another).
The forces that cause adhesion and cohesion can be ...
and/or
biocompatibility
Biocompatibility is related to the behavior of biomaterials in various contexts. The term refers to the ability of a material to perform with an appropriate host response in a specific situation. The ambiguity of the term reflects the ongoing de ...
See also
*
Ion beam
An ion beam is a type of charged particle beam consisting of ions. Ion beams have many uses in electronics manufacturing (principally ion implantation) and other industries. A variety of ion beam sources exists, some derived from the mercury ...
*
RF antenna ion source
*
On-Line Isotope Mass Separator
The ISOLDE Radioactive Ion Beam Facility, is an on-line isotope separator facility located at the heart of the CERN accelerator complex on the Franco-Swiss border. The name of the facility is an acronym for Isotope Separator On Line DEvice. Cre ...
References
{{authority control
Ions
Accelerator physics
 An ion source is a device that creates atomic and molecular
An ion source is a device that creates atomic and molecular 
 These ion sources use a or
These ion sources use a or 
 Plasma desorption ionization mass spectrometry (PDMS), also called fission fragment ionization, is a mass spectrometry technique in which ionization of material in a solid sample is accomplished by bombarding it with ionic or neutral atoms formed as a result of the
Plasma desorption ionization mass spectrometry (PDMS), also called fission fragment ionization, is a mass spectrometry technique in which ionization of material in a solid sample is accomplished by bombarding it with ionic or neutral atoms formed as a result of the  Spray ionization methods involve the formation of aerosol particles from a liquid solution and the formation of bare ions after solvent evaporation.
Solvent-assisted ionization (SAI) is a method in which charged droplets are produced by introducing a solution containing analyte into a heated inlet tube of an atmospheric pressure ionization mass spectrometer. Just as in Electrospray Ionization (ESI), desolvation of the charged droplets produces multiply charged analyte ions. Volatile and nonvolatile compounds are analyzed by SAI, and high voltage is not required to achieve sensitivity comparable to ESI. Application of a voltage to the solution entering the hot inlet through a zero dead volume fitting connected to fused silica tubing produces ESI-like mass spectra, but with higher sensitivity. The inlet tube to the mass spectrometer becomes the ion source.
Spray ionization methods involve the formation of aerosol particles from a liquid solution and the formation of bare ions after solvent evaporation.
Solvent-assisted ionization (SAI) is a method in which charged droplets are produced by introducing a solution containing analyte into a heated inlet tube of an atmospheric pressure ionization mass spectrometer. Just as in Electrospray Ionization (ESI), desolvation of the charged droplets produces multiply charged analyte ions. Volatile and nonvolatile compounds are analyzed by SAI, and high voltage is not required to achieve sensitivity comparable to ESI. Application of a voltage to the solution entering the hot inlet through a zero dead volume fitting connected to fused silica tubing produces ESI-like mass spectra, but with higher sensitivity. The inlet tube to the mass spectrometer becomes the ion source.

 In
In 
 In
In  Gas flows through the ion source between the
Gas flows through the ion source between the