Hydrogen embrittlement on:
[Wikipedia]
[Google]
[Amazon]
 Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the
Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the
 Hydrogen embrittlement is a complex process involving a number of distinct contributing micro-mechanisms, not all of which need to be present. The mechanisms include the formation of brittle
Hydrogen embrittlement is a complex process involving a number of distinct contributing micro-mechanisms, not all of which need to be present. The mechanisms include the formation of brittle
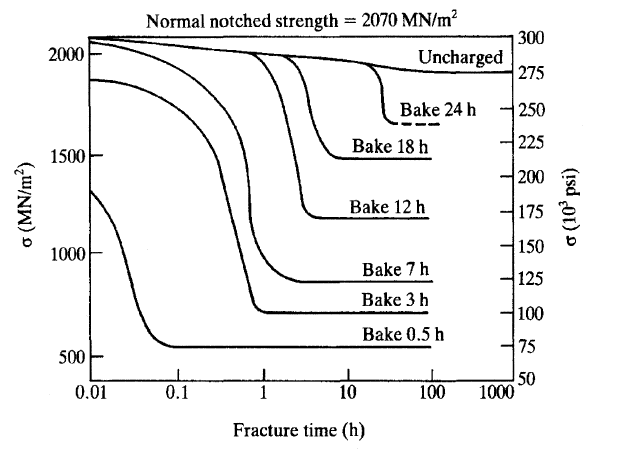 Steel with an ultimate
Steel with an ultimate
Resources on hydrogen embrittlement, Cambridge University
Hydrogen embrittlement
Hydrogen purity plays a critical role
A Sandia National Lab technical reference manual.
Hydrogen embrittlement, NASA
{{DEFAULTSORT:Hydrogen Embrittlement Corrosion Electrochemistry Hydrogen Materials degradation Metalworking
 Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the
Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the ductility
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile str ...
of a metal due to absorbed hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
. Hydrogen atoms are small and can permeate
In physics and engineering, permeation (also called imbuing) is the penetration of a permeate (a fluid such as a liquid, gas, or vapor) through a solid. It is directly related to the concentration gradient of the permeate, a material's intrins ...
solid metals. Once absorbed, hydrogen lowers the stress
Stress may refer to:
Science and medicine
* Stress (biology), an organism's response to a stressor such as an environmental condition
* Stress (linguistics), relative emphasis or prominence given to a syllable in a word, or to a word in a phrase ...
required for cracks in the metal to initiate and propagate, resulting in embrittlement. Hydrogen embrittlement occurs most notably in steels, as well as in iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
, titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
, cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
, and their alloys. Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
, aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
, and stainless steels are less susceptible to hydrogen embrittlement.
The essential facts about the nature of hydrogen embrittlement have been known since the 19th century.
Hydrogen embrittlement is maximised at around room temperature in steels, and most metals are relatively immune to hydrogen embrittlement at temperatures above 150 °C. Hydrogen embrittlement requires the presence of both atomic ("diffusible") hydrogen and a mechanical stress
In continuum mechanics, stress is a physical quantity. It is a quantity that describes the magnitude of forces that cause deformation. Stress is defined as ''force per unit area''. When an object is pulled apart by a force it will cause elonga ...
to induce crack growth, although that stress may be applied or residual. Hydrogen embrittlement increases at lower strain rate
In materials science, strain rate is the change in strain ( deformation) of a material with respect to time.
The strain rate at some point within the material measures the rate at which the distances of adjacent parcels of the material change ...
s. In general, higher-strength materials are more susceptible to hydrogen embrittlement.
Metals can be exposed to hydrogen from two types of sources: gaseous hydrogen and hydrogen chemically generated at the metal surface. Gaseous hydrogen is molecular hydrogen and does not cause embrittlement though it can cause hot hydrogen attack (see below). It is the atomic hydrogen from chemical attack which causes embrittlement because the atomic hydrogen dissolves quickly into the metal at room temperature. Gaseous hydrogen is found in pressure vessels and pipelines. Electrochemical
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outc ...
sources of hydrogen include acids (as may be encountered during pickling
Pickling is the process of preserving or extending the shelf life of food by either anaerobic fermentation in brine or immersion in vinegar. The pickling procedure typically affects the food's texture and flavor. The resulting food is cal ...
, etching
Etching is traditionally the process of using strong acid or mordant to cut into the unprotected parts of a metal surface to create a design in intaglio (incised) in the metal. In modern manufacturing, other chemicals may be used on other types ...
, or cleaning), corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
(typically due to aqueous corrosion or cathodic protection), and electroplating. Hydrogen can be introduced into the metal during manufacturing by the presence of moisture during welding
Welding is a fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing fusion. Welding is distinct from lower temperature techniques such as bra ...
or while the metal is molten. The most common causes of failure in practice are poorly-controlled electroplating or damp welding rods
Plastic welding is welding for semi-finished plastic materials, and is described in ISO 472 as a process of uniting softened surfaces of materials, generally with the aid of heat (except solvent welding). Welding of thermoplastics is accomplishe ...
.
Hydrogen embrittlement as a term can be used to refer specifically to the embrittlement that occurs in steels and similar metals at relatively low hydrogen concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', ''molar concentration'', '' number concentration'', ...
s, or it can be used to encompass all embrittling effects that hydrogen has on metals. These broader embrittling effects include hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
formation, which occurs in titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
and vanadium but not in steels, and hydrogen-induced blistering, which only occurs at high hydrogen concentrations and does not require the presence of stress. However, hydrogen embrittlement is almost always distinguished from high temperature hydrogen attack (HTHA), which occurs in steels at temperatures above 400 °C and involves the formation of methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ea ...
pockets. As of 2016, the mechanism by which hydrogen causes embrittlement in steels is not fully understood and continues to be debated.
Mechanisms
 Hydrogen embrittlement is a complex process involving a number of distinct contributing micro-mechanisms, not all of which need to be present. The mechanisms include the formation of brittle
Hydrogen embrittlement is a complex process involving a number of distinct contributing micro-mechanisms, not all of which need to be present. The mechanisms include the formation of brittle hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
s, the creation of voids that can lead to high-pressure bubbles, enhanced decohesion at internal surfaces and localised plasticity at crack tips that assist in the propagation of cracks. There is a great variety of mechanisms that have been proposed and investigated as to the cause of brittleness once diffusible hydrogen has been dissolved into the metal. In recent years, it has become widely accepted that HE is a complex, material and environmental dependent process, so that no single mechanism applies exclusively.
* Internal pressure: At high hydrogen concentrations, absorbed hydrogen species recombine in voids to form hydrogen molecules (H2), creating pressure from within the metal. This pressure can increase to levels where cracks form, commonly designated hydrogen-induced cracking (HIC), as well as blisters forming on the specimen surface, designated hydrogen-induced blistering. These effects can reduce ductility
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile str ...
and tensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials t ...
.
* Hydrogen enhanced localised plasticity (HELP): Hydrogen increases the nucleation and movement of dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to s ...
s at a crack tip. HELP results in crack propagation by localised ductile failure at the crack tip with less deformation
Deformation can refer to:
* Deformation (engineering), changes in an object's shape or form due to the application of a force or forces.
** Deformation (physics), such changes considered and analyzed as displacements of continuum bodies.
* Defor ...
occurring in the surrounding material, which gives a brittle
A material is brittle if, when subjected to stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of high strength. Br ...
appearance to the fracture.
* Hydrogen decreased dislocation emission: Molecular dynamics
Molecular dynamics (MD) is a computer simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic "evolution" of t ...
simulations reveal a ductile-to-brittle transition caused by the suppression of dislocation emission at the crack tip by dissolved hydrogen. This prevents the crack tip rounding-off, so the sharp crack then leads to brittle-cleavage failure.
* Hydrogen enhanced decohesion (HEDE): Interstitial hydrogen lowers the stress required for metal atoms to fracture apart. HEDE can only occur when the local concentration of hydrogen is high, such as due to the increased hydrogen solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solub ...
in the tensile stress
In continuum mechanics, stress is a physical quantity. It is a quantity that describes the magnitude of forces that cause deformation. Stress is defined as ''force per unit area''. When an object is pulled apart by a force it will cause elonga ...
field at a crack tip, at stress concentrators, or in the tension field of edge dislocations.
* Metal hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
formation: The formation of brittle hydrides with the parent material allows cracks to propagate in a brittle fashion. This is particularly a problem with vanadium alloys, but most structural alloys do not easily form hydrides.
* Phase transformations: Hydrogen can induce phase transformations in some materials, and the new phase may be less ductile.
Material susceptibility
Hydrogen embrittles a variety of metals including steel,aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
(at high temperatures only), and titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
. Austempered iron is also susceptible, though austempered steel (and possibly other austempered metals) displays increased resistance to hydrogen embrittlement. NASA has reviewed which metals are susceptible to embrittlement and which only prone to hot hydrogen attack: nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
alloys, austenitic stainless steels, aluminium and alloys, copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
(including alloys, e.g. beryllium copper). Sandia has also produced a comprehensive guide.
Steels
 Steel with an ultimate
Steel with an ultimate tensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials t ...
of less than 1000 MPa (~145,000 psi) or hardness of less than HRC 32 on the Hardness Rockwell Scale is not generally considered susceptible to hydrogen embrittlement. As an example of severe hydrogen embrittlement, the elongation at failure of 17-4PH precipitation hardened stainless steel was measured to drop from 17% to only 1.7% when smooth specimens were exposed to high-pressure hydrogen.
As the strength of steels increases, the fracture toughness decreases, so the likelihood that hydrogen embrittlement will lead to fracture increases. In high-strength steels, anything above a hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. In general, different materials differ in their hardness; for example hard ...
of HRC 32 may be susceptible to early hydrogen cracking after plating
Plating is a surface covering in which a metal is deposited on a conductive surface. Plating has been done for hundreds of years; it is also critical for modern technology. Plating is used to decorate objects, for corrosion inhibition, to impro ...
processes that introduce hydrogen. They may also experience long-term failures anytime from weeks to decades after being placed in service due to accumulation of hydrogen over time from cathodic protection and other sources. Numerous failures have been reported in the hardness range from HRC 32-36 and more above; therefore, parts in this range should be checked during quality control to ensure they are not susceptible.
Copper
Copper alloys which containoxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
can be embrittled if exposed to hot hydrogen. The hydrogen diffuses through the copper and reacts with inclusions of , forming 2 metallic Cu atoms and (water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
), which then forms pressurized bubbles at the grain boundaries
In materials science, a grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are two-dimensional defects in the crystal structure, and tend to decrease the electrical and thermal ...
. This process can cause the grains to literally be forced away from each other, and is known as ''steam embrittlement'' (because steam is directly produced inside the copper crystal lattice, not because exposure of copper to external steam causes the problem).
Vanadium, nickel, and titanium
Alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
s of vanadium, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
, and titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
have a high hydrogen solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solub ...
, and can therefore absorb significant amounts of hydrogen. This can lead to hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride ...
formation, resulting in irregular volume expansion and reduced ductility
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile str ...
(because metallic hydrides are fragile ceramic materials
A ceramic is any of the various hard, brittle, heat-resistant and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, ...
). This is a particular issue when looking for non-palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
-based alloys for use in hydrogen separation membranes.
Fatigue
While most failures in practice have been through fast failure, there is experimental evidence that hydrogen also affects the fatigue properties of steels. This is entirely expected given the nature of the embrittlement mechanisms proposed for fast fracture. In general hydrogen embrittlement has a strong effect on high-stress
Stress may refer to:
Science and medicine
* Stress (biology), an organism's response to a stressor such as an environmental condition
* Stress (linguistics), relative emphasis or prominence given to a syllable in a word, or to a word in a phrase ...
, low-cycle fatigue and very little effect on high-cycle fatigue.
Sources of hydrogen
During manufacture, hydrogen can be dissolved into the component by processes such as phosphating,pickling
Pickling is the process of preserving or extending the shelf life of food by either anaerobic fermentation in brine or immersion in vinegar. The pickling procedure typically affects the food's texture and flavor. The resulting food is cal ...
, electroplating, casting
Casting is a manufacturing process in which a liquid material is usually poured into a mold, which contains a hollow cavity of the desired shape, and then allowed to solidify. The solidified part is also known as a ''casting'', which is ejected ...
, carbonizing
Carbonization is the conversion of organic matters like plants and dead animal remains into carbon through destructive distillation.
Complexity in carbonization
Carbonization is a pyrolytic reaction, therefore, is considered a complex process ...
, surface cleaning, electrochemical machining, welding
Welding is a fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing fusion. Welding is distinct from lower temperature techniques such as bra ...
, hot roll forming, and heat treatments.
During service use, hydrogen can be dissolved into the metal from wet corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
or through misapplication of protection measures such as cathodic protection. In one case of failure during construction of the San Francisco–Oakland Bay Bridge galvanized
Galvanization or galvanizing ( also spelled galvanisation or galvanising) is the process of applying a protective zinc coating to steel or iron, to prevent rusting. The most common method is hot-dip galvanizing, in which the parts are submerged ...
(i.e. zinc-plated) rods were left wet for 5 years before being tensioned. The reaction of the zinc with water introduced hydrogen into the steel.
A common case of embrittlement during manufacture is poor arc welding
Arc welding is a welding process that is used to join metal to metal by using electricity to create enough heat to melt metal, and the melted metals, when cool, result in a binding of the metals. It is a type of welding that uses a welding powe ...
practice, in which hydrogen is released from moisture, such as in the coating of welding electrodes or from damp welding rods
Plastic welding is welding for semi-finished plastic materials, and is described in ISO 472 as a process of uniting softened surfaces of materials, generally with the aid of heat (except solvent welding). Welding of thermoplastics is accomplishe ...
. To avoid atomic hydrogen formation in the high temperature plasma of the arc, welding rods have to be perfectly dried in an oven at the appropriate temperature and duration before use. Another way to minimize the formation of hydrogen is to use special low-hydrogen electrodes for welding high-strength steels.
Apart from arc welding, the most common problems are from chemical or electrochemical processes which, by reduction of hydrogen ions or water, generate hydrogen atoms at the surface, which rapidly dissolve in the metal. One of these chemical reactions involves hydrogen sulfide () in sulfide stress cracking (SSC), a significant problem for the oil and gas industries.
After a manufacturing process or treatment which may cause hydrogen ingress, the component should be baked to remove or immobilize the hydrogen.
Prevention
Hydrogen embrittlement can be prevented through several methods, all of which are centered on minimizing contact between the metal and hydrogen, particularly during fabrication and theelectrolysis of water
Electrolysis of water, also known as electrochemical water splitting, is the process of using electricity to decompose water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, or remi ...
. Embrittling procedures such as acid pickling should be avoided, as should increased contact with elements such as sulfur and phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
. The use of proper electroplating solution and procedures can also help to prevent hydrogen embrittlement.
If the metal has not yet started to crack, hydrogen embrittlement can be reversed by removing the hydrogen source and causing the hydrogen within the metal to diffuse out through heat treatment
Heat treating (or heat treatment) is a group of industrial process, industrial, thermal and metalworking, metalworking processes used to alter the physical property, physical, and sometimes chemical property, chemical, properties of a material. ...
. This de-embrittlement process, known as low hydrogen annealing or "baking", is used to overcome the weaknesses of methods such as electroplating which introduce hydrogen to the metal, but is not always entirely effective because a sufficient time and temperature must be reached. Tests such as ASTM F1624 can be used to rapidly identify the minimum baking time (by testing using design of experiments
The design of experiments (DOE, DOX, or experimental design) is the design of any task that aims to describe and explain the variation of information under conditions that are hypothesized to reflect the variation. The term is generally associ ...
, a relatively low number of samples can be used to pinpoint this value). Then the same test can be used as a quality control check to evaluate if baking was sufficient on a per-batch basis.
In the case of welding, often pre-heating and post-heating the metal is applied to allow the hydrogen to diffuse out before it can cause any damage. This is specifically done with high-strength steels and low alloy steel
Alloy steel is steel that is alloyed with a variety of elements in total amounts between 1.0% and 50% by weight to improve its mechanical properties. Alloy steels are broken down into two groups: low alloy steels and high alloy steels. The differe ...
s such as the chromium/ molybdenum/ vanadium alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
s. Due to the time needed to re-combine hydrogen atoms into the hydrogen molecules, hydrogen cracking due to welding can occur over 24 hours after the welding operation is completed.
Another way of preventing this problem is through materials selection. This will build an inherent resistance to this process and reduce the need of post processing or constant monitoring for failure. Certain metals or alloys are highly susceptible to this issue, so choosing a material that is minimally affected while retaining the desired properties would also provide an optimal solution. Much research has been done to catalog the compatibility of certain metals with hydrogen. Tests such as ASTM F1624 can also be used to rank alloys and coatings during materials selection to ensure (for instance) that the threshold of cracking is below the threshold for hydrogen-assisted stress corrosion cracking. Similar tests can also be used during quality control to more effectively qualify materials being produced in a rapid and comparable manner.
Testing
Most analytical methods for hydrogen embrittlement involve evaluating the effects of (1) internal hydrogen from production and/or (2) external sources of hydrogen such as cathodic protection. For steels, it is important to test specimens in the lab that are at least as hard (or harder) than the final parts will be. Ideally, specimens should be made of the final material or the nearest possible representative, as fabrication can have a profound impact on resistance to hydrogen-assisted cracking. There are numerous ASTM standards for testing for hydrogen embrittlement: * ASTM B577 is the ''Standard Test Methods for Detection of Cuprous Oxide (Hydrogen Embrittlement Susceptibility) in Copper''. The test focuses on hydrogen embrittlement of copper alloys, including a metallographic evaluation (method A), testing in a hydrogen charged chamber followed by metallography (method B), and method C is the same as B but includes a bend test. * ASTM B839 is the ''Standard Test Method for Residual Embrittlement in Metallic Coated, Externally Threaded Articles, Fasteners, and Rod-Inclined Wedge Method''. * ASTM F519 is the ''Standard Test Method for Mechanical Hydrogen Embrittlement Evaluation of Plating/Coating Processes and Service Environments''. There are 7 different samples designs and the two most commons tests are (1) the rapid test, the Rising step load testing (RSL) method per ASTM F1624 and (2) the sustained load test, which takes 200 hours. The sustained load test is still included in many legacy standards, but the RSL method is increasingly being adopted due to speed, repeatability, and the quantitative nature of the test. The RSL method provides an accurate ranking of the effect of hydrogen from both internal and external sources. * ASTM F1459 is the ''Standard Test Method for Determination of the Susceptibility of Metallic Materials to Hydrogen Gas Embrittlement (HGE) Test''. The test uses a diaphragm loaded with a differential pressure. * ASTM G142 is the ''Standard Test Method for Determination of Susceptibility of Metals to Embrittlement in Hydrogen Containing Environments at High Pressure, High Temperature, or Both''. The test uses a cylindrical tensile specimen tested into an enclosure pressurized with hydrogen orhelium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
.
* ASTM F1624 is the ''Standard Test Method for Measurement of Hydrogen Embrittlement Threshold in Steel by the Incremental Step Loading Technique''. The test uses the incremental step loading (ISL) or Rising step load testing (RSL) method for quantitatively testing for the Hydrogen Embrittlement threshold stress for the onset of Hydrogen-Induced Cracking due to platings and coatings from Internal Hydrogen Embrittlement (IHE) and Environmental Hydrogen Embrittlement (EHE). F1624 provides a rapid, quantitative measure of the effects of hydrogen both from internal sources and external sources (which is accomplished by applying a selected voltage in an electrochemical cell). The F1624 test is performed by comparing a standard fast-fracture tensile strength to the fracture strength from a Rising step load testing practice where the load is held for hour(s) at each step. In many cases it can be performed in 30 hours or less.
* ASTM F1940 is the ''Standard Test Method for Process Control Verification to Prevent Hydrogen Embrittlement in Plated or Coated Fasteners''. While the title now explicitly includes the word fasteners, F1940 was not originally intended for these purposes. F1940 is based on the F1624 method and is similar to F519 but with different root radius and stress concentration factors. When specimens exhibit a threshold cracking of 75% of the net fracture strength, the plating bath is considered to be 'non-embrittling'.
There are many other related standards for hydrogen embrittlement:
* NACE TM0284-2003 (NACE International
The Association for Materials Protection and Performance (AMPP), is a professional association focused on the protection of assets and performance of materials. AMPP was created when NACE International and SSPC the Society for Protective Coat ...
) Resistance to Hydrogen-Induced Cracking
* ISO 11114-4:2005 (ISO
ISO is the most common abbreviation for the International Organization for Standardization.
ISO or Iso may also refer to: Business and finance
* Iso (supermarket), a chain of Danish supermarkets incorporated into the SuperBest chain in 2007
* Iso ...
)Test methods for selecting metallic materials resistant to hydrogen embrittlement.
* Standard Test Method for Mechanical Hydrogen Embrittlement Evaluation of Plating/Coating Processes and Service Environments
Notable failures from hydrogen embrittlement
* In 2013, six months prior to opening, the East Span of the Oakland Bay Bridge failed during testing. Catastrophic failures occurred in shear bolts in the span, after only two weeks of service, with the failure attributed to embrittlement (see details above). * In theCity of London
The City of London is a city, ceremonial county and local government district that contains the historic centre and constitutes, alongside Canary Wharf, the primary central business district (CBD) of London. It constituted most of London f ...
, 122 Leadenhall Street
122 Leadenhall Street, which is also known as the Leadenhall Building, is a skyscraper in central London. It opened in July 2014 and was designed by the Rogers Stirk Harbour + Partners; it is known informally as The Cheesegrater because of its ...
, generally known as 'the Cheesegrater', suffered from hydrogen embrittlement in steel bolts, with three bolts failing in 2014 and 2015. Most of the 3,000 bolts were replaced at a cost of £6m.
See also
* Hydrogen analyzer * Hydrogen damage * Hydrogen piping * Hydrogen safety * Low hydrogen annealing * Nascent hydrogen *Oxygen-free copper
Oxygen-free copper (OFC) or oxygen-free high thermal conductivity (OFHC) copper is a group of wrought high-conductivity copper alloys that have been electrolytically refined to reduce the level of oxygen to 0.001% or below.
Specification
Ox ...
* Stress corrosion cracking
References
External links
Resources on hydrogen embrittlement, Cambridge University
Hydrogen embrittlement
Hydrogen purity plays a critical role
A Sandia National Lab technical reference manual.
Hydrogen embrittlement, NASA
{{DEFAULTSORT:Hydrogen Embrittlement Corrosion Electrochemistry Hydrogen Materials degradation Metalworking