
The history of chemistry represents a time span from
ancient history
Ancient history is a time period from the beginning of writing and recorded human history to as far as late antiquity. The span of recorded history is roughly 5,000 years, beginning with the Sumerian cuneiform script. Ancient history cove ...
to the present. By 1000 BC, civilizations used technologies that would eventually form the basis of the various branches of chemistry. Examples include the discovery of fire, extracting
metal
A metal (from ancient Greek, Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, e ...
s from
ores, making
pottery
Pottery is the process and the products of forming vessels and other objects with clay and other ceramic materials, which are fired at high temperatures to give them a hard and durable form. Major types include earthenware, stoneware and ...
and glazes, fermenting
beer
Beer is one of the oldest and the most widely consumed type of alcoholic drink in the world, and the third most popular drink overall after water and tea. It is produced by the brewing and fermentation of starches, mainly derived from ce ...
and
wine
Wine is an alcoholic drink typically made from Fermentation in winemaking, fermented grapes. Yeast in winemaking, Yeast consumes the sugar in the grapes and converts it to ethanol and carbon dioxide, releasing heat in the process. Different ...
, extracting chemicals from plants for
medicine
Medicine is the science and practice of caring for a patient, managing the diagnosis, prognosis, prevention, treatment, palliation of their injury or disease, and promoting their health. Medicine encompasses a variety of health care pr ...
and
perfume
Perfume (, ; french: parfum) is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent ...
, rendering fat into
soap
Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products. In a domestic setting, soaps are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are us ...
, making
glass
Glass is a non- crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenchin ...
,
and making
alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductili ...
s like
bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals, such as phosphorus, or metalloids suc ...
.
The protoscience of chemistry,
alchemy
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim wo ...
, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. While both
alchemy
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim wo ...
and
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, proper ...
are concerned with matter and its transformations,
chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe th ...
s are seen as applying
scientific method
The scientific method is an empirical method for acquiring knowledge that has characterized the development of science since at least the 17th century (with notable practitioners in previous centuries; see the article history of scientifi ...
to their work.
The history of chemistry is intertwined with the
history of thermodynamics
The history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general. Owing to the relevance of thermodynamics in much of science and technology, its history is finel ...
, especially through the work of
Willard Gibbs
Josiah Willard Gibbs (; February 11, 1839 – April 28, 1903) was an American scientist who made significant theoretical contributions to physics, chemistry, and mathematics. His work on the applications of thermodynamics was instrumental in ...
.
Ancient history
Early humans
A 100,000-year-old
ochre
Ochre ( ; , ), or ocher in American English, is a natural clay earth pigment, a mixture of ferric oxide and varying amounts of clay and sand. It ranges in colour from yellow to deep orange or brown. It is also the name of the colours produced ...
-processing workshop was found at
Blombos Cave in
South Africa
South Africa, officially the Republic of South Africa (RSA), is the southernmost country in Africa. It is bounded to the south by of coastline that stretch along the South Atlantic and Indian Oceans; to the north by the neighbouring coun ...
. It indicates that early humans had an elementary knowledge of chemistry. Paintings drawn by early humans consisting of early humans mixing animal blood with other liquids found on cave walls also indicate a small knowledge of chemistry.
Early metallurgy
The earliest recorded metal employed by humans seems to be
gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile ...
, which can be found free or "native". Small amounts of natural gold have been found in Spanish caves used during the late
Paleolithic
The Paleolithic or Palaeolithic (), also called the Old Stone Age (from Greek: παλαιός '' palaios'', "old" and λίθος ''lithos'', "stone"), is a period in human prehistory that is distinguished by the original development of stone too ...
period, around 40,000 BC.
Silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
,
copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
,
tin and
meteoric iron can also be found native, allowing a limited amount of
metalworking
Metalworking is the process of shaping and reshaping metals to create useful objects, parts, assemblies, and large scale structures. As a term it covers a wide and diverse range of processes, skills, and tools for producing objects on every scale ...
in ancient cultures.
[Photos, E., 'The Question of Meteorictic versus Smelted Nickel-Rich Iron: Archaeological Evidence and Experimental Results' ''World Archaeology'' Vol. 20, No. 3, Archaeometallurgy (February 1989), pp. 403–421]
Online version
accessed on 2010-02-08. Egyptian weapons made from meteoric iron in about 3000 BC were highly prized as "daggers from Heaven".
[W. Keller (1963) ''The Bible as History'', p. 156 ]
Arguably the first chemical reaction used in a controlled manner was
fire
Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction products.
At a certain point in the combustion reaction, called the ignition point, flames ...
. However, for millennia fire was seen simply as a mystical force that could transform one substance into another (burning wood, or boiling water) while producing heat and light. Fire affected many aspects of early societies. These ranged from the simplest facets of everyday life, such as cooking and habitat heating and lighting, to more advanced uses, such as making pottery and bricks and melting of metals to make tools.
It was fire that led to the discovery of
glass
Glass is a non- crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenchin ...
and the
purification of metals; this was followed by the rise of
metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
Metallurgy encompasses both the sc ...
. During the early stages of metallurgy, methods of purification of metals were sought, and gold, known in
ancient Egypt as early as 2900 BC, became a precious metal.
Bronze Age
Certain metals can be recovered from their ores by simply heating the rocks in a fire: notably
tin,
lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, ...
and (at a higher temperature) copper. This process is known as
smelting
Smelting is a process of applying heat to ore, to extract a base metal. It is a form of extractive metallurgy. It is used to extract many metals from their ores, including silver, iron, copper, and other base metals. Smelting uses heat and a c ...
. The first evidence of this extractive metallurgy dates from the 6th and 5th millennia BC, and was found in the archaeological sites of the
Vinča culture,
Majdanpek,
Jarmovac and
Pločnik in
Serbia
Serbia (, ; Serbian: , , ), officially the Republic of Serbia ( Serbian: , , ), is a landlocked country in Southeastern and Central Europe, situated at the crossroads of the Pannonian Basin and the Balkans. It shares land borders with Hu ...
. To date, the earliest copper smelting is found at the Belovode site; these examples include a copper axe from 5500 BC. Other signs of early metals are found from the third millennium BC in places like
Palmela
Palmela () is a town and a municipality in Portugal. The population in 2011 was 62,831, in an area of 465.12 km².
The municipality is located in the Lisboa Region and Setúbal District, about south of Lisbon. The municipal holiday is 1 Ju ...
(Portugal),
Los Millares (Spain), and
Stonehenge
Stonehenge is a prehistoric monument on Salisbury Plain in Wiltshire, England, west of Amesbury. It consists of an outer ring of vertical sarsen standing stones, each around high, wide, and weighing around 25 tons, topped by connec ...
(United Kingdom). However, as often happens in the study of
prehistoric times, the ultimate beginnings cannot be clearly defined and new discoveries are ongoing.

These first metals were single elements, or else combinations as naturally occurred. By combining copper and tin, a superior metal could be made, an
alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductili ...
called
bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals, such as phosphorus, or metalloids suc ...
. This was a major technological shift that began the
Bronze Age
The Bronze Age is a historic period, lasting approximately from 3300 BC to 1200 BC, characterized by the use of bronze, the presence of writing in some areas, and other early features of urban civilization. The Bronze Age is the second pri ...
about 3500 BC. The Bronze Age was a period in human cultural development when the most advanced metalworking (at least in systematic and widespread use) included techniques for smelting
copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
and
tin from naturally occurring outcroppings of copper ores, and then smelting those ores to cast bronze. These naturally occurring ores typically included arsenic as a common impurity. Copper/tin ores are rare, as reflected in the absence of tin bronzes in
western Asia
Western Asia, West Asia, or Southwest Asia, is the westernmost subregion of the larger geographical region of Asia, as defined by some academics, UN bodies and other institutions. It is almost entirely a part of the Middle East, and includes A ...
before 3000 BC.
After the Bronze Age, the history of metallurgy was marked by armies seeking better weaponry. States in
Eurasia
Eurasia (, ) is the largest continental area on Earth, comprising all of Europe and Asia. Primarily in the Northern and Eastern Hemispheres, it spans from the British Isles and the Iberian Peninsula in the west to the Japanese archipelag ...
prospered when they made the superior alloys, which, in turn, made better armor and better weapons. Significant progress in metallurgy and alchemy was made in
ancient India
According to consensus in modern genetics, anatomically modern humans first arrived on the Indian subcontinent from Africa between 73,000 and 55,000 years ago. Quote: "Y-Chromosome and Mt-DNA data support the colonization of South Asia by ...
.
Iron Age
The extraction of
iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
from its ore into a workable metal is much more difficult than copper or tin. While iron is not better suited for tools than bronze (until
steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistan ...
was discovered), iron ore is much more abundant and common than either copper or tin, and therefore more often available locally, with no need to trade for it.
Iron working appears to have been invented by the
Hittites
The Hittites () were an Anatolian people who played an important role in establishing first a kingdom in Kussara (before 1750 BC), then the Kanesh or Nesha kingdom (c. 1750–1650 BC), and next an empire centered on Hattusa in north-cent ...
in about 1200 BC, beginning the
Iron Age
The Iron Age is the final epoch of the three-age division of the prehistory and protohistory of humanity. It was preceded by the Stone Age ( Paleolithic, Mesolithic, Neolithic) and the Bronze Age ( Chalcolithic). The concept has been mostly ...
. The secret of extracting and working iron was a key factor in the success of the
Philistines
The Philistines ( he, פְּלִשְׁתִּים, Pəlīštīm; Koine Greek ( LXX): Φυλιστιείμ, romanized: ''Phulistieím'') were an ancient people who lived on the south coast of Canaan from the 12th century BC until 604 BC, whe ...
.
[
The Iron Age refers to the advent of iron working ( ferrous metallurgy). Historical developments in ferrous metallurgy can be found in a wide variety of past cultures and civilizations. These include the ancient and medieval kingdoms and empires of the Middle East and Near East, ancient Iran, ancient Egypt, ancient ]Nubia
Nubia () ( Nobiin: Nobīn, ) is a region along the Nile river encompassing the area between the first cataract of the Nile (just south of Aswan in southern Egypt) and the confluence of the Blue and White Niles (in Khartoum in central Sud ...
, and Anatolia
Anatolia, tr, Anadolu Yarımadası), and the Anatolian plateau, also known as Asia Minor, is a large peninsula in Western Asia and the westernmost protrusion of the Asian continent. It constitutes the major part of modern-day Turkey. The re ...
(Turkey), Ancient Nok, Carthage
Carthage was the capital city of Ancient Carthage, on the eastern side of the Lake of Tunis in what is now Tunisia. Carthage was one of the most important trading hubs of the Ancient Mediterranean and one of the most affluent cities of the classi ...
, the Greeks
The Greeks or Hellenes (; el, Έλληνες, ''Éllines'' ) are an ethnic group and nation indigenous to the Eastern Mediterranean and the Black Sea regions, namely Greece, Cyprus, Albania, Italy, Turkey, Egypt, and, to a lesser extent, ot ...
and Roman
Roman or Romans most often refers to:
* Rome, the capital city of Italy
* Ancient Rome, Roman civilization from 8th century BC to 5th century AD
*Roman people, the people of ancient Rome
*''Epistle to the Romans'', shortened to ''Romans'', a lett ...
s of ancient Europe, medieval Europe, ancient and medieval China, ancient and medieval India, ancient and medieval Japan, amongst others. Many applications, practices, and devices associated with or involved in metallurgy were established in ancient China, such as the innovation of the blast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being "forced" or supplied above atmospheri ...
, cast iron
Cast iron is a class of iron– carbon alloys with a carbon content more than 2%. Its usefulness derives from its relatively low melting temperature. The alloy constituents affect its color when fractured: white cast iron has carbide impuri ...
, hydraulic-powered trip hammers, and double-acting piston bellows.[Temple, Robert K.G. (2007). ''The Genius of China: 3,000 Years of Science, Discovery, and Invention'' (3rd edition). London: André Deutsch. pp. 44–56. .]
Classical antiquity and atomism
 Philosophical attempts to rationalize why different substances have different properties (color, density, smell), exist in different states (gaseous, liquid, and solid), and react in a different manner when exposed to environments, for example to water or fire or temperature changes, led ancient philosophers to postulate the first theories on nature and chemistry. The history of such philosophical theories that relate to chemistry can probably be traced back to every single ancient civilization. The common aspect in all these theories was the attempt to identify a small number of primary classical elements that make up all the various substances in nature. Substances like air, water, and soil/earth, energy forms, such as fire and light, and more abstract concepts such as thoughts, aether, and heaven, were common in ancient civilizations even in the absence of any cross-fertilization: for example ancient Greek, Indian, Mayan, and Chinese philosophies all considered air,
Philosophical attempts to rationalize why different substances have different properties (color, density, smell), exist in different states (gaseous, liquid, and solid), and react in a different manner when exposed to environments, for example to water or fire or temperature changes, led ancient philosophers to postulate the first theories on nature and chemistry. The history of such philosophical theories that relate to chemistry can probably be traced back to every single ancient civilization. The common aspect in all these theories was the attempt to identify a small number of primary classical elements that make up all the various substances in nature. Substances like air, water, and soil/earth, energy forms, such as fire and light, and more abstract concepts such as thoughts, aether, and heaven, were common in ancient civilizations even in the absence of any cross-fertilization: for example ancient Greek, Indian, Mayan, and Chinese philosophies all considered air, water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
, earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's sur ...
and fire
Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction products.
At a certain point in the combustion reaction, called the ignition point, flames ...
as primary elements.
Ancient world
Around 420 BC, Empedocles
Empedocles (; grc-gre, Ἐμπεδοκλῆς; , 444–443 BC) was a Greek pre-Socratic philosopher and a native citizen of Akragas, a Greek city in Sicily. Empedocles' philosophy is best known for originating the cosmogonic theory of the ...
stated that all matter is made up of four elemental substances: earth, fire, air and water. The early theory of atomism
Atomism (from Greek , ''atomon'', i.e. "uncuttable, indivisible") is a natural philosophy proposing that the physical universe is composed of fundamental indivisible components known as atoms.
References to the concept of atomism and its atom ...
can be traced back to ancient Greece
Ancient Greece ( el, Ἑλλάς, Hellás) was a northeastern Mediterranean civilization, existing from the Greek Dark Ages of the 12th–9th centuries BC to the end of classical antiquity ( AD 600), that comprised a loose collection of cu ...
and ancient India
According to consensus in modern genetics, anatomically modern humans first arrived on the Indian subcontinent from Africa between 73,000 and 55,000 years ago. Quote: "Y-Chromosome and Mt-DNA data support the colonization of South Asia by ...
.[ Will Durant (1935), ''Our Oriental Heritage'':
]
Greek atomism was made popular by the Greek philosopher Democritus
Democritus (; el, Δημόκριτος, ''Dēmókritos'', meaning "chosen of the people"; – ) was an Ancient Greek pre-Socratic philosopher from Abdera, primarily remembered today for his formulation of an atomic theory of the universe. No ...
, who declared that matter is composed of indivisible and indestructible particles called "atomos" around 380 BC. Earlier, Leucippus also declared that atoms were the most indivisible part of matter (this coincided with a similar declaration by Indian
Indian or Indians may refer to:
Peoples South Asia
* Indian people, people of Indian nationality, or people who have an Indian ancestor
** Non-resident Indian, a citizen of India who has temporarily emigrated to another country
* South Asia ...
philosopher Kanada in his Vaisheshika sutra
''Sutra'' ( sa, सूत्र, translit=sūtra, translit-std=IAST, translation=string, thread)Monier Williams, ''Sanskrit English Dictionary'', Oxford University Press, Entry fo''sutra'' page 1241 in Indian literary traditions refers to an ap ...
s around the same time period).[ ]Aristotle
Aristotle (; grc-gre, Ἀριστοτέλης ''Aristotélēs'', ; 384–322 BC) was a Greek philosopher and polymath during the Classical period in Ancient Greece. Taught by Plato, he was the founder of the Peripatetic school of ...
opposed the existence of atoms in 330 BC. A Greek text attributed to Polybus the physician (ca. 380 BC) argued that the human body is composed of four humours
Humorism, the humoral theory, or humoralism, was a system of medicine detailing a supposed makeup and workings of the human body, adopted by Ancient Greek and Roman physicians and philosophers.
Humorism began to fall out of favor in the 1850s ...
instead. Epicurus
Epicurus (; grc-gre, Ἐπίκουρος ; 341–270 BC) was an ancient Greek philosopher and sage who founded Epicureanism, a highly influential school of philosophy. He was born on the Greek island of Samos to Athenian parents. Influence ...
(fl. 300 BC) postulated a universe of indestructible atoms in which man himself is responsible for achieving a balanced life.
With the goal of explaining Epicurean philosophy
Epicureanism is a system of philosophy founded around 307 BC based upon the teachings of the ancient Greek philosopher Epicurus. Epicureanism was originally a challenge to Platonism. Later its main opponent became Stoicism.
Few writings by Epi ...
to a Roman audience, the Roman
Roman or Romans most often refers to:
* Rome, the capital city of Italy
* Ancient Rome, Roman civilization from 8th century BC to 5th century AD
*Roman people, the people of ancient Rome
*''Epistle to the Romans'', shortened to ''Romans'', a lett ...
poet and philosopher Lucretius
Titus Lucretius Carus ( , ; – ) was a Roman poet and philosopher. His only known work is the philosophical poem '' De rerum natura'', a didactic work about the tenets and philosophy of Epicureanism, and which usually is translated into E ...
wrote ''De rerum natura
''De rerum natura'' (; ''On the Nature of Things'') is a first-century BC didactic poem by the Roman poet and philosopher Lucretius ( – c. 55 BC) with the goal of explaining Epicurean philosophy to a Roman audience. The poem, written in some ...
'' (The Nature of Things) in 50 BC. In the work, Lucretius presents the principles of atomism
Atomism (from Greek , ''atomon'', i.e. "uncuttable, indivisible") is a natural philosophy proposing that the physical universe is composed of fundamental indivisible components known as atoms.
References to the concept of atomism and its atom ...
; the nature of the mind
The mind is the set of faculties responsible for all mental phenomena. Often the term is also identified with the phenomena themselves. These faculties include thought, imagination, memory, will, and sensation. They are responsible for various m ...
and soul
In many religious and philosophical traditions, there is a belief that a soul is "the immaterial aspect or essence of a human being".
Etymology
The Modern English noun '' soul'' is derived from Old English ''sāwol, sāwel''. The earliest att ...
; explanations of sensation
Sensation (psychology) refers to the processing of the senses by the sensory system.
Sensation or sensations may also refer to:
In arts and entertainment In literature
* Sensation (fiction), a fiction writing mode
* Sensation novel, a Briti ...
and thought; the development of the world and its phenomena; and explains a variety of celestial and terrestrial phenomena.
The earliest alchemists in the Western tradition seemed to have come from Greco-Roman Egypt
The history of Egypt has been long and wealthy, due to the flow of the Nile River with its fertile banks and delta, as well as the accomplishments of Egypt's native inhabitants and outside influence. Much of Egypt's ancient history was a myster ...
in the first centuries AD. In addition to technical work, many of them invented chemical apparatuses. The ''bain-marie'', or water bath, is named for Mary the Jewess. Her work also gives the first descriptions of the ''tribikos'' and ''kerotakis''. Cleopatra the Alchemist described furnaces and has been credited with the invention of the alembic
An alembic (from ar, الإنبيق, al-inbīq, originating from grc, ἄμβιξ, ambix, 'cup, beaker') is an alchemical still consisting of two vessels connected by a tube, used for distillation of liquids.
Description
The complete dis ...
. Later, Zosimos of Panopolis wrote books on alchemy, which he called ''cheirokmeta'', the Greek word for "things made by hand." These works include many references to recipes and procedures, as well as descriptions of instruments. Much of the early development of purification methods were described earlier by Pliny the Elder
Gaius Plinius Secundus (AD 23/2479), called Pliny the Elder (), was a Roman author, naturalist and natural philosopher, and naval and army commander of the early Roman Empire, and a friend of the emperor Vespasian. He wrote the encyclopedic ' ...
in his Naturalis Historia. He tried to explain those methods, as well as making acute observations of the state of many minerals.
Medieval alchemy
 The elemental system used in medieval
The elemental system used in medieval alchemy
Alchemy (from Arabic: ''al-kīmiyā''; from Ancient Greek: χυμεία, ''khumeía'') is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practiced in China, India, the Muslim wo ...
was developed primarily by the Persian-Arab
The Arabs (singular: Arab; singular ar, عَرَبِيٌّ, DIN 31635: , , plural ar, عَرَب, DIN 31635: , Arabic pronunciation: ), also known as the Arab people, are an ethnic group mainly inhabiting the Arab world in Western Asia, ...
alchemist Jābir ibn Hayyān and was rooted in the classical elements of Greek tradition. His system consisted of the four Aristotelian elements of air, earth, fire, and water in addition to two philosophical elements: sulphur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
, characterizing the principle of combustibility, "the stone which burns"; and mercury, characterizing the principle of metallic properties. They were seen by early alchemists as idealized expressions of irreducible components of the universe
The universe is all of space and time and their contents, including planets, stars, galaxies, and all other forms of matter and energy. The Big Bang theory is the prevailing cosmological description of the development of the univers ...
and are of larger consideration within philosophical alchemy.
The three metallic principles (sulphur to flammability or combustion, mercury to volatility and stability, and salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quant ...
to solidity) became the ''tria prima'' of the Swiss alchemist Paracelsus
Paracelsus (; ; 1493 – 24 September 1541), born Theophrastus von Hohenheim (full name Philippus Aureolus Theophrastus Bombastus von Hohenheim), was a Swiss physician, alchemist, lay theologian, and philosopher of the German Renaissance.
He ...
. He reasoned that Aristotle's four-element theory appeared in bodies as three principles. Paracelsus saw these principles as fundamental and justified them by recourse to the description of how wood burns in fire. Mercury included the cohesive principle, so that when it left the wood (in smoke) the wood fell apart. Smoke described the volatility (the mercurial principle), the heat-giving flames described flammability (sulphur), and the remnant ash described solidity (salt).
The philosopher's stone
 Alchemy is defined by the Hermetic quest for the philosopher's stone, the study of which is steeped in symbolic mysticism, and differs greatly from modern science. Alchemists toiled to make transformations on an
Alchemy is defined by the Hermetic quest for the philosopher's stone, the study of which is steeped in symbolic mysticism, and differs greatly from modern science. Alchemists toiled to make transformations on an esoteric
Western esotericism, also known as esotericism, esoterism, and sometimes the Western mystery tradition, is a term scholars use to categorise a wide range of loosely related ideas and movements that developed within Western society. These ideas ...
(spiritual) and/or exoteric
Exoteric refers to knowledge that is outside and independent from a person's experience and can be ascertained by anyone (related to common sense).
The word is derived from the comparative form of Greek ἔξω ''eksô'', "from, out of, outside" ...
(practical) level. It was the protoscientific, exoteric aspects of alchemy that contributed heavily to the evolution of chemistry in Greco-Roman Egypt
The history of Egypt has been long and wealthy, due to the flow of the Nile River with its fertile banks and delta, as well as the accomplishments of Egypt's native inhabitants and outside influence. Much of Egypt's ancient history was a myster ...
, in the Islamic Golden Age
The Islamic Golden Age was a period of cultural, economic, and scientific flourishing in the history of Islam, traditionally dated from the 8th century to the 14th century. This period is traditionally understood to have begun during the reign ...
, and then in Europe. Alchemy and chemistry share an interest in the composition and properties of matter, and until the 18th century they were not separate disciplines. The term ''chymistry'' has been used to describe the blend of alchemy and chemistry that existed before that time.
During the Renaissance, exoteric alchemy remained popular in the form of Paracelsian
Paracelsianism (also Paracelsism; German: ') was an early modern medical movement based on the theories and therapies of Paracelsus.
It developed in the second half of the 16th century, during the decades following Paracelsus' death in 1541, an ...
iatrochemistry, while spiritual alchemy flourished, realigned to its Platonic, Hermetic, and Gnostic
Gnosticism (from grc, γνωστικός, gnōstikós, , 'having knowledge') is a collection of religious ideas and systems which coalesced in the late 1st century AD among Jewish and early Christian sects. These various groups emphasized p ...
roots. Consequently, the symbolic quest for the philosopher's stone was not superseded by scientific advances, and was still the domain of respected scientists and doctors until the early 18th century. Early modern alchemists who are renowned for their scientific contributions include Jan Baptist van Helmont, Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders ...
, and Isaac Newton
Sir Isaac Newton (25 December 1642 – 20 March 1726/27) was an English mathematician, physicist, astronomer, alchemist, Theology, theologian, and author (described in his time as a "natural philosophy, natural philosopher"), widely ...
.
Alchemy in the Islamic world
In the Islamic World, the Muslim
Muslims ( ar, المسلمون, , ) are people who adhere to Islam, a monotheistic religion belonging to the Abrahamic tradition. They consider the Quran, the foundational religious text of Islam, to be the verbatim word of the God of Abrah ...
s were translating the works of ancient Greek and Hellenistic
In Classical antiquity, the Hellenistic period covers the time in Mediterranean history after Classical Greece, between the death of Alexander the Great in 323 BC and the emergence of the Roman Empire, as signified by the Battle of Actium in ...
philosophers into Arabic and were experimenting with scientific ideas. The Arabic works attributed to the 8th-century alchemist Jābir ibn Hayyān introduced a systematic classification of chemical substances, and provided instructions for deriving an inorganic compound ( sal ammoniac or ammonium chloride) from organic substances (such as plants, blood, and hair) by chemical means. Some Arabic Jabirian works (e.g., the "Book of Mercy", and the "Book of Seventy") were later translated into Latin under the Latinized name "Geber", and in 13th-century Europe an anonymous writer, usually referred to as pseudo-Geber, started to produce alchemical and metallurgical writings under this name. Later influential Muslim philosophers, such as Abū al-Rayhān al-Bīrūnī and Avicenna
Ibn Sina ( fa, ابن سینا; 980 – June 1037 CE), commonly known in the West as Avicenna (), was a Persian polymath who is regarded as one of the most significant physicians, astronomers, philosophers, and writers of the Islamic ...
disputed the theories of alchemy, particularly the theory of the transmutation of metals.
Problems encountered with alchemy
There were several problems with alchemy, as seen from today's standpoint. There was no systematic naming scheme for new compounds, and the language was esoteric and vague to the point that the terminologies meant different things to different people. In fact, according to ''The Fontana History of Chemistry'' (Brock, 1992):
The language of alchemy soon developed an arcane and secretive technical vocabulary designed to conceal information from the uninitiated. To a large degree, this language is incomprehensible to us today, though it is apparent that readers of Geoffery Chaucer's Canon's Yeoman's Tale or audiences of Ben Jonson
Benjamin "Ben" Jonson (c. 11 June 1572 – c. 16 August 1637) was an English playwright and poet. Jonson's artistry exerted a lasting influence upon English poetry and stage comedy. He popularised the comedy of humours; he is best known for t ...
's The Alchemist
An alchemist is a person who practices alchemy.
Alchemist or Alchemyst may also refer to:
Books and stories
* ''The Alchemist'' (novel), the translated title of a 1988 allegorical novel by Paulo Coelho
* ''The Alchemist'' (play), a play by Be ...
were able to construe it sufficiently to laugh at it.
Chaucer's tale exposed the more fraudulent side of alchemy, especially the manufacture of counterfeit gold from cheap substances. Less than a century earlier, Dante Alighieri
Dante Alighieri (; – 14 September 1321), probably baptized Durante di Alighiero degli Alighieri and often referred to as Dante (, ), was an Italian poet, writer and philosopher. His '' Divine Comedy'', originally called (modern Italian: ...
also demonstrated an awareness of this fraudulence, causing him to consign all alchemists to the Inferno in his writings. Soon afterwards, in 1317, the Avignon
Avignon (, ; ; oc, Avinhon, label= Provençal or , ; la, Avenio) is the prefecture of the Vaucluse department in the Provence-Alpes-Côte d'Azur region of Southeastern France. Located on the left bank of the river Rhône, the commune had ...
Pope John XXII
Pope John XXII ( la, Ioannes PP. XXII; 1244 – 4 December 1334), born Jacques Duèze (or d'Euse), was head of the Catholic Church from 7 August 1316 to his death in December 1334.
He was the second and longest-reigning Avignon Pope, elected b ...
ordered all alchemists to leave France for making counterfeit money. A law was passed in England in 1403 which made the "multiplication of metals" punishable by death. Despite these and other apparently extreme measures, alchemy did not die. Royalty and privileged classes still sought to discover the philosopher's stone and the elixir of life for themselves.
There was also no agreed-upon scientific method for making experiments reproducible. Indeed, many alchemists included in their methods irrelevant information such as the timing of the tides or the phases of the moon. The esoteric nature and codified vocabulary of alchemy appeared to be more useful in concealing the fact that they could not be sure of very much at all. As early as the 14th century, cracks seemed to grow in the facade of alchemy; and people became sceptical. Clearly, there needed to be a scientific method in which experiments could be repeated by other people, and results needed to be reported in a clear language that laid out both what was known and what was unknown.
17th and 18th centuries: Early chemistry
 Practical attempts to improve the refining of ores and their extraction to smelt metals was an important source of information for early chemists in the 16th century, among them
Practical attempts to improve the refining of ores and their extraction to smelt metals was an important source of information for early chemists in the 16th century, among them Georg Agricola
Georgius Agricola (; born Georg Pawer or Georg Bauer; 24 March 1494 – 21 November 1555) was a German Humanist scholar, mineralogist and metallurgist. Born in the small town of Glauchau, in the Electorate of Saxony of the Holy Roman Empir ...
(1494–1555), who published his great work '' De re metallica'' in 1556. His work describes the highly developed and complex processes of mining metal ores, metal extraction and metallurgy of the time. His approach removed the mysticism associated with the subject, creating the practical base upon which others could build. The work describes the many kinds of furnace used to smelt ore, and stimulated interest in minerals and their composition. It is no coincidence that he gives numerous references to the earlier author, Pliny the Elder and his ''Naturalis Historia''. Agricola has been described as the "father of metallurgy".
In 1605, Sir Francis Bacon published ''The Proficience and Advancement of Learning'', which contains a description of what would later be known as the scientific method
The scientific method is an empirical method for acquiring knowledge that has characterized the development of science since at least the 17th century (with notable practitioners in previous centuries; see the article history of scientifi ...
. In 1605, Michal Sedziwój
Michael Sendivogius (; pl, Michał Sędziwój; 2 February 1566 – 1636) was a Polish alchemist, philosopher, and medical doctor. A pioneer of chemistry, he developed ways of purification and creation of various acids, metals and other chemi ...
publishes the alchemical treatise ''A New Light of Alchemy'' which proposed the existence of the "food of life" within air, much later recognized as oxygen. In 1615 Jean Beguin published the ''Tyrocinium Chymicum
''Tyrocinium Chymicum'' was a published set of chemistry lecture notes started by Jean Beguin in 1610 in Paris, France. It has been cited as the first chemistry textbook (as opposed to that for alchemy
Alchemy (from Arabic: ''al-kīmiyā''; ...
'', an early chemistry textbook, and in it draws the first-ever chemical equation. In 1637 René Descartes
René Descartes ( or ; ; Latinized: Renatus Cartesius; 31 March 1596 – 11 February 1650) was a French philosopher, scientist, and mathematician, widely considered a seminal figure in the emergence of modern philosophy and science. Ma ...
publishes '' Discours de la méthode'', which contains an outline of the scientific method.
The Dutch chemist Jan Baptist van Helmont's work ''Ortus medicinae'' was published posthumously in 1648; the book is cited by some as a major transitional work between alchemy and chemistry, and as an important influence on Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders ...
. The book contains the results of numerous experiments and establishes an early version of the law of conservation of mass
In physics and chemistry, the law of conservation of mass or principle of mass conservation states that for any system closed to all transfers of matter and energy, the mass of the system must remain constant over time, as the system's mass can ...
. Working during the time just after Paracelsus
Paracelsus (; ; 1493 – 24 September 1541), born Theophrastus von Hohenheim (full name Philippus Aureolus Theophrastus Bombastus von Hohenheim), was a Swiss physician, alchemist, lay theologian, and philosopher of the German Renaissance.
He ...
and iatrochemistry, Jan Baptist van Helmont suggested that there are insubstantial substances other than air and coined a name for them – " gas", from the Greek word ''chaos''. In addition to introducing the word "gas" into the vocabulary of scientists, van Helmont conducted several experiments involving gases. Jan Baptist van Helmont is also remembered today largely for his ideas on spontaneous generation and his 5-year tree experiment, as well as being considered the founder of pneumatic chemistry.
Robert Boyle

 Anglo-Irish chemist
Anglo-Irish chemist Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders ...
(1627–1691) is considered to have initiated the gradual separation of chemistry from alchemy. Although skeptical of elements and convinced of alchemy, Boyle played a key part in elevating the "sacred art" as an independent, fundamental and philosophical discipline. He is best known for Boyle's law, which he presented in 1662, though he was not the first to discover it.pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country a ...
and volume
Volume is a measure of occupied three-dimensional space. It is often quantified numerically using SI derived units (such as the cubic metre and litre) or by various imperial or US customary units (such as the gallon, quart, cubic inch). ...
of a gas, if the temperature is kept constant within a closed system.[Levine, Ira. N. (1978), p12 gives the original definition.]
Boyle is also credited for his landmark publication '' The Sceptical Chymist'' (1661), which advocated for a rigorous approach to experimentation among chemists. In the work, Boyle questioned some commonly held alchemical theories and argued for practitioners to be more “philosophical” and less commercially focused. He rejected the classical four elements of earth, fire, air, and water, and proposed a mechanistic alternative of atoms and chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking ...
s that could be subject to rigorous experiment.
Boyle also tried to purify chemicals to obtain reproducible reactions. He was a vocal proponent of the mechanical philosophy proposed by René Descartes
René Descartes ( or ; ; Latinized: Renatus Cartesius; 31 March 1596 – 11 February 1650) was a French philosopher, scientist, and mathematician, widely considered a seminal figure in the emergence of modern philosophy and science. Ma ...
to explain and quantify the physical properties and interactions of material substances. Boyle was an atomist, but favoured the word ''corpuscle'' over ''atoms''. He commented that the finest division of matter where the properties are retained is at the level of corpuscles.
Boyle repeated the tree experiment of van Helmont, and was the first to use indicators
Indicator may refer to:
Biology
* Environmental indicator of environmental health (pressures, conditions and responses)
* Ecological indicator of ecosystem health (ecological processes)
* Health indicator, which is used to describe the health o ...
which changed colors with acidity. He also performed numerous investigations with an air pump
An air pump is a pump for pushing air. Examples include a bicycle pump, pumps that are used to aerate an aquarium or a pond via an airstone; a gas compressor used to power a pneumatic tool, air horn or pipe organ; a bellows used to encourage a ...
, and noted that the mercury fell as air was pumped out. He also observed that pumping the air out of a container would extinguish a flame and kill small animals placed inside. Through his works, Boyle helped to lay the foundations for the chemical revolution two centuries later.
Development and dismantling of phlogiston
 In 1702, German chemist Georg Stahl coined the name " phlogiston" for the substance believed to be released in the process of burning. Around 1735, Swedish chemist Georg Brandt analyzed a dark blue pigment found in copper ore. Brandt demonstrated that the pigment contained a new element, later named
In 1702, German chemist Georg Stahl coined the name " phlogiston" for the substance believed to be released in the process of burning. Around 1735, Swedish chemist Georg Brandt analyzed a dark blue pigment found in copper ore. Brandt demonstrated that the pigment contained a new element, later named cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
. In 1751, a Swedish chemist and pupil of Stahl's named Axel Fredrik Cronstedt, identified an impurity in copper ore as a separate metallic element, which he named nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow t ...
. Cronstedt is one of the founders of modern mineralogy
Mineralogy is a subject of geology specializing in the scientific study of the chemistry, crystal structure, and physical (including optical) properties of minerals and mineralized artifacts. Specific studies within mineralogy include the proce ...
. Cronstedt also discovered the mineral scheelite in 1751, which he named tungsten, meaning "heavy stone" in Swedish.
In 1754, Scottish chemist Joseph Black isolated carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
, which he called "fixed air". In 1757, Louis Claude Cadet de Gassicourt, while investigating arsenic compounds, creates Cadet's fuming liquid, later discovered to be cacodyl oxide, considered to be the first synthetic organometallic compound. In 1758, Joseph Black formulated the concept of latent heat to explain the thermochemistry of phase changes. In 1766, English chemist Henry Cavendish isolated hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
, which he called "inflammable air". Cavendish discovered hydrogen as a colorless, odourless gas that burns and can form an explosive mixture with air, and published a paper on the production of water by burning inflammable air (that is, hydrogen) in dephlogisticated air (now known to be oxygen), the latter a constituent of atmospheric air (phlogiston theory
The phlogiston theory is a superseded scientific theory that postulated the existence of a fire-like element called phlogiston () contained within combustible bodies and released during combustion. The name comes from the Ancient Greek (''bur ...
).
In 1773, Swedish chemist Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydr ...
discovered oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
, which he called "fire air", but did not immediately publish his achievement. In 1774, English chemist Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted ...
independently isolated oxygen in its gaseous state, calling it "dephlogisticated air", and published his work before Scheele. During his lifetime, Priestley's considerable scientific reputation rested on his invention of soda water, his writings on electricity
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as describe ...
, and his discovery of several "airs" (gases), the most famous being what Priestley dubbed "dephlogisticated air" (oxygen). However, Priestley's determination to defend phlogiston theory and to reject what would become the chemical revolution eventually left him isolated within the scientific community.
In 1781, Carl Wilhelm Scheele discovered that a new acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a se ...
, tungstic acid
Tungstic acid refers to hydrated forms of tungsten trioxide, WO3. Both a monohydrate (WO3·H2O) and hemihydrate (WO3·1/2 H2O) are known. Molecular species akin to sulfuric acid, i.e. (HO)2WO2 are not observed.
The solid-state structure of ...
, could be made from Cronstedt's scheelite (at the time named tungsten). Scheele and Torbern Bergman suggested that it might be possible to obtain a new metal by reducing this acid.José
José is a predominantly Spanish and Portuguese form of the given name Joseph. While spelled alike, this name is pronounced differently in each language: Spanish ; Portuguese (or ).
In French, the name ''José'', pronounced , is an old vernac ...
and Fausto Elhuyar found an acid made from wolframite that was identical to tungstic acid. Later that year, in Spain, the brothers succeeded in isolating the metal now known as tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
by reduction of this acid with charcoal
Charcoal is a lightweight black carbon residue produced by strongly heating wood (or other animal and plant materials) in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, ...
, and they are credited with the discovery of the element.
Volta and the Voltaic pile
Italian physicist Alessandro Volta
Alessandro Giuseppe Antonio Anastasio Volta (, ; 18 February 1745 – 5 March 1827) was an Italian physicist, chemist and lay Catholic who was a pioneer of electricity and power who is credited as the inventor of the electric battery and th ...
constructed a device for accumulating a large charge by a series of inductions and groundings. He investigated the 1780s discovery " animal electricity" by Luigi Galvani
Luigi Galvani (, also ; ; la, Aloysius Galvanus; 9 September 1737 – 4 December 1798) was an Italian physician, physicist, biologist and philosopher, who studied animal electricity. In 1780, he discovered that the muscles of dead frogs' legs ...
, and found that the electric current
An electric current is a stream of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is measured as the net rate of flow of electric charge through a surface or into a control volume. The movi ...
was generated from the contact of dissimilar metals, and that the frog leg was only acting as a detector. Volta demonstrated in 1794 that when two metals and brine-soaked cloth or cardboard are arranged in a circuit they produce an electric current.
In 1800, Volta stacked several pairs of alternating copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pink ...
(or silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
) and zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
discs (electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials ...
s) separated by cloth or cardboard soaked in brine (electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon ...
) to increase the electrolyte conductivity.electrical battery
An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices.
When a battery is supplying power, its positive terminal is the cathode and its nega ...
to produce electricity
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as describe ...
.
Thus, Volta is considered to be the founder of the discipline of electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an out ...
. A Galvanic cell (or voltaic cell) is an electrochemical cell that derives electrical energy from a spontaneous redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
reaction taking place within the cell. It generally consists of two different metals connected by a salt bridge, or individual half-cells separated by a porous membrane.
Antoine-Laurent de Lavoisier
 Antoine-Laurent de Lavoisier demonstrated with careful measurements that transmutation of water to earth was not possible, but that the sediment observed from boiling water came from the container. He burnt phosphorus and sulfur in air, and proved that the products weighed more than the original samples, with the mass gained being lost from the air. Thus, in 1789, he established the Law of Conservation of Mass, which is also called "Lavoisier's Law."
Repeating the experiments of Priestley, he demonstrated that air is composed of two parts, one of which combines with metals to form calxes. In ''Considérations Générales sur la Nature des Acides'' (1778), he demonstrated that the "air" responsible for combustion was also the source of acidity. The next year, he named this portion oxygen (Greek for acid-former), and the other azote (Greek for no life). Because of his more thorough characterization of it as an element, Lavoisier thus has a claim to the discovery of oxygen along with Priestley and Scheele. He also discovered that the "inflammable air" discovered by Cavendish – which he termed
Antoine-Laurent de Lavoisier demonstrated with careful measurements that transmutation of water to earth was not possible, but that the sediment observed from boiling water came from the container. He burnt phosphorus and sulfur in air, and proved that the products weighed more than the original samples, with the mass gained being lost from the air. Thus, in 1789, he established the Law of Conservation of Mass, which is also called "Lavoisier's Law."
Repeating the experiments of Priestley, he demonstrated that air is composed of two parts, one of which combines with metals to form calxes. In ''Considérations Générales sur la Nature des Acides'' (1778), he demonstrated that the "air" responsible for combustion was also the source of acidity. The next year, he named this portion oxygen (Greek for acid-former), and the other azote (Greek for no life). Because of his more thorough characterization of it as an element, Lavoisier thus has a claim to the discovery of oxygen along with Priestley and Scheele. He also discovered that the "inflammable air" discovered by Cavendish – which he termed hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
(Greek for water-former) – combined with oxygen to produce a dew, as Priestley had reported, which appeared to be water. In ''Reflexions sur le Phlogistique'' (1783), Lavoisier showed the phlogiston theory
The phlogiston theory is a superseded scientific theory that postulated the existence of a fire-like element called phlogiston () contained within combustible bodies and released during combustion. The name comes from the Ancient Greek (''bur ...
of combustion to be inconsistent. Mikhail Lomonosov independently established a tradition of chemistry in Russia in the 18th century; he also rejected the phlogiston theory, and anticipated the kinetic theory of gases
Kinetic (Ancient Greek: κίνησις “kinesis”, movement or to move) may refer to:
* Kinetic theory, describing a gas as particles in random motion
* Kinetic energy, the energy of an object that it possesses due to its motion
Art and ent ...
. Lomonosov regarded heat as a form of motion, and stated the idea of conservation of matter.
Lavoisier worked with Claude Louis Berthollet and others to devise a system of chemical nomenclature, which serves as the basis of the modern system of naming chemical compounds. In his ''Methods of Chemical Nomenclature'' (1787), Lavoisier invented the system of naming and classification still largely in use today, including names such as sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
, sulfates, and sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid ( sulfurous acid) is elusive, its salts are w ...
s. In 1785, Berthollet was the first to introduce the use of chlorine gas as a commercial bleach. In the same year he first determined the elemental composition of the gas ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
. Berthollet first produced a modern bleaching liquid in 1789 by passing chlorine gas through a solution of sodium carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions ...
– the result was a weak solution of sodium hypochlorite. Another strong chlorine oxidant and bleach which he investigated and was the first to produce, potassium chlorate
Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO3. In its pure form, it is a white crystalline substance. After sodium chlorate, it is the second most common chlorate in industrial use. ...
(KClO3), is known as Berthollet's Salt. Berthollet is also known for his scientific contributions to the theory of chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the ...
via the mechanism of reversible reaction
A reversible reaction is a reaction in which the conversion of reactants to products and the conversion of products to reactants occur simultaneously.
: \mathit aA + \mathit bB \mathit cC + \mathit dD
A and B can react to form C and D or, in the ...
s.
Lavoisier's ''Traité Élémentaire de Chimie
''Traité élémentaire de chimie'' (''Elementary Treatise on Chemistry'') is a textbook written by Antoine Lavoisier published in 1789 and translated into English by Robert Kerr in 1790 under the title ''Elements of Chemistry in a New Systemati ...
'' (Elementary Treatise of Chemistry, 1789) was the first modern chemical textbook, and presented a unified view of new theories of chemistry, contained a clear statement of the Law of Conservation of Mass, and denied the existence of phlogiston. In addition, it contained a list of elements, or substances that could not be broken down further, which included oxygen, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, hydrogen, phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
, mercury, zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
, and sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
. His list, however, also included light and caloric, which he believed to be material substances. In the work, Lavoisier underscored the observational basis of his chemistry, stating "I have tried...to arrive at the truth by linking up facts; to suppress as much as possible the use of reasoning, which is often an unreliable instrument which deceives us, in order to follow as much as possible the torch of observation and of experiment." Nevertheless, he believed that the real existence of atoms was philosophically impossible. Lavoisier demonstrated that organisms disassemble and reconstitute atmospheric air in the same manner as a burning body.
With Pierre-Simon Laplace
Pierre-Simon, marquis de Laplace (; ; 23 March 1749 – 5 March 1827) was a French scholar and polymath whose work was important to the development of engineering, mathematics, statistics, physics, astronomy, and philosophy. He summarize ...
, Lavoisier used a calorimeter to estimate the heat evolved per unit of carbon dioxide produced. They found the same ratio for a flame and animals, indicating that animals produced energy by a type of combustion. Lavoisier believed in the radical theory, which stated that radicals, which function as a single group in a chemical reaction, would combine with oxygen in reactions. He believed all acids contained oxygen. He also discovered that diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, b ...
is a crystalline form of carbon.
Although many of Lavoisier's partners were influential for the advancement of chemistry as a scientific discipline, his wife Marie-Anne Lavoisier was arguably the most influential of them all. Upon their marriage, Mme. Lavoisier began to study chemistry, English, and drawing in order to help her husband in his work either by translating papers into English, a language which Lavoisier did not know, or by keeping records and drawing the various apparatuses that Lavoisier used in his labs.French Revolution
The French Revolution ( ) was a period of radical political and societal change in France that began with the Estates General of 1789 and ended with the formation of the French Consulate in November 1799. Many of its ideas are conside ...
.
19th century
In 1802, French American chemist and industrialist Éleuthère Irénée du Pont
Éleuthère Irénée du Pont de Nemours (; ; 24 June 1771 – 31 October 1834) was a French-American chemist and industrialist who founded the gunpowder manufacturer E. I. du Pont de Nemours and Company. His descendants, the du Pont family, hav ...
, who learned manufacture of gunpowder and explosives under Antoine Lavoisier, founded a gunpowder manufacturer in Delaware known as E. I. du Pont de Nemours and Company
DuPont de Nemours, Inc., commonly shortened to DuPont, is an American multinational chemical company first formed in 1802 by French-American chemist and industrialist Éleuthère Irénée du Pont de Nemours. The company played a major role in ...
. The French Revolution
The French Revolution ( ) was a period of radical political and societal change in France that began with the Estates General of 1789 and ended with the formation of the French Consulate in November 1799. Many of its ideas are conside ...
forced his family to move to the United States where du Pont started a gunpowder mill on the Brandywine River in Delaware. Wanting to make the best powder possible, du Pont was vigilant about the quality of the materials he used. For 32 years, du Pont served as president of E. I. du Pont de Nemours and Company, which eventually grew into one of the largest and most successful companies in America.
Throughout the 19th century, chemistry was divided between those who followed the atomic theory of John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He is best known for introducing the atomic theory into chemistry, and for his research into Color blindness, colour blindness, which ...
and those who did not, such as Wilhelm Ostwald and Ernst Mach
Ernst Waldfried Josef Wenzel Mach ( , ; 18 February 1838 – 19 February 1916) was a Moravian-born Austrian physicist and philosopher, who contributed to the physics of shock waves. The ratio of one's speed to that of sound is named the Mach n ...
.[
] Although such proponents of the atomic theory as Amedeo Avogadro and Ludwig Boltzmann made great advances in explaining the behavior of gases, this dispute was not finally settled until Jean Perrin
Jean Baptiste Perrin (30 September 1870 – 17 April 1942) was a French physicist who, in his studies of the Brownian motion of minute particles suspended in liquids (sedimentation equilibrium), verified Albert Einstein’s explanation of this p ...
's experimental investigation of Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born Theoretical physics, theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for d ...
's atomic explanation of Brownian motion in the first decade of the 20th century.Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English scientist who contributed to the study of electromagnetism and electrochemistry. His main discoveries include the principles underlying electromagnetic inducti ...
was another early worker, whose major contribution to chemistry was electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an out ...
, in which (among other things) a certain quantity of electricity during electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
or electrodeposition of metals was shown to be associated with certain quantities of chemical elements, and fixed quantities of the elements therefore with each other, in specific ratios. These findings, like those of Dalton's combining ratios, were early clues to the atomic nature of matter.

Vladimir Markovnikov
Vladimir Markovnikov born in 1838, was a Russian Scientist who proceeded most of his work at Kazan University in Russia.
John Dalton
 In 1803, English meteorologist and chemist
In 1803, English meteorologist and chemist John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He is best known for introducing the atomic theory into chemistry, and for his research into Color blindness, colour blindness, which ...
proposed Dalton's law, which describes the relationship between the components in a mixture of gases and the relative pressure each contributes to that of the overall mixture.atomic theory
Atomic theory is the scientific theory that matter is composed of particles called atoms. Atomic theory traces its origins to an ancient philosophical tradition known as atomism. According to this idea, if one were to take a lump of matter ...
in 1803 which stated that all matter was composed of small indivisible particles termed atoms, atoms of a given element possess unique characteristics and weight, and three types of atoms exist: simple (elements), compound (simple molecules), and complex (complex molecules). In 1808, Dalton first published ''New System of Chemical Philosophy'' (1808–1827), in which he outlined the first modern scientific description of the atomic theory. This work identified chemical elements as a specific type of atom, therefore rejecting Newton's theory of chemical affinities.
Instead, Dalton inferred proportions of elements in compounds by taking ratios of the weights of reactants, setting the atomic weight of hydrogen to be identically one. Following Jeremias Benjamin Richter (known for introducing the term ''stoichiometry
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equ ...
''), he proposed that chemical elements combine in integral ratios. This is known as the law of multiple proportions or Dalton's law, and Dalton included a clear description of the law in his ''New System of Chemical Philosophy''. The law of multiple proportions is one of the basic laws of stoichiometry used to establish the atomic theory. Despite the importance of the work as the first view of atoms as physically real entities and the introduction of a system of chemical symbols, ''New System of Chemical Philosophy'' devoted almost as much space to the caloric theory as to atomism.
French chemist Joseph Proust proposed the law of definite proportions, which states that elements always combine in small, whole number ratios to form compounds, based on several experiments conducted between 1797 and 1804 Along with the law of multiple proportions, the law of definite proportions forms the basis of stoichiometry. The law of definite proportions and constant composition do not prove that atoms exist, but they are difficult to explain without assuming that chemical compounds are formed when atoms combine in constant proportions.
Jöns Jacob Berzelius
 A Swedish chemist and disciple of Dalton,
A Swedish chemist and disciple of Dalton, Jöns Jacob Berzelius
Baron Jöns Jacob Berzelius (; by himself and his contemporaries named only Jacob Berzelius, 20 August 1779 – 7 August 1848) was a Swedish chemist. Berzelius is considered, along with Robert Boyle, John Dalton, and Antoine Lavoisier, to be o ...
embarked on a systematic program to try to make accurate and precise quantitative measurements and to ensure the purity of chemicals. Along with Lavoisier, Boyle, and Dalton, Berzelius is known as the father of modern chemistry. In 1828 he compiled a table of relative atomic weights, where oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
was used as a standard, with its weight set at 100, and which included all of the elements known at the time. This work provided evidence in favor of Dalton's atomic theory – that inorganic chemical compounds are composed of atoms combined in whole number amounts. He determined the exact elementary constituents of a large number of compounds; the results strongly supported Proust's Law of Definite Proportions. In discovering that atomic weights are not integer multiples of the weight of hydrogen, Berzelius also disproved Prout's hypothesis that elements are built up from atoms of hydrogen.
Motivated by his extensive atomic weight determinations and in a desire to aid his experiments, he introduced the classical system of chemical symbols and notation with his 1808 publication ''Lärbok i Kemien'', in which elements are abbreviated to one or two letters to make a distinct symbol from their Latin name. This system of chemical notation—in which the elements were given simple written labels, such as O for oxygen, or Fe for iron, with proportions denoted by numbers—is the same basic system used today. The only difference is that instead of the subscript number used today (e.g., H2O), Berzelius used a superscript (H2O). Berzelius is credited with identifying the chemical elements silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
, selenium, thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
, and cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
. Students working in Berzelius's laboratory also discovered lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense soli ...
and vanadium.
Berzelius developed the radical theory of chemical combination, which holds that reactions occur as stable groups of atoms called radicals
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
are exchanged between molecules. He believed that salts are compounds formed of acids and bases, and discovered that the anions in acids were attracted to a positive electrode (the anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ...
), whereas the cations in a base were attracted to a negative electrode (the cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
). Berzelius did not believe in the Vitalism
Vitalism is a belief that starts from the premise that "living organisms are fundamentally different from non-living entities because they contain some non-physical element or are governed by different principles than are inanimate things." Wher ...
Theory, but instead in a regulative force which produced organization of tissues in an organism. Berzelius is also credited with originating the chemical terms "catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
", "polymer
A polymer (; Greek '' poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
", "isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Is ...
", and "allotrope
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical State of matter, state, known as allotropes of the elements. Allotropes are different structural modifications o ...
", although his original definitions differ dramatically from modern usage. For example, he coined the term "polymer" in 1833 to describe organic compounds which shared identical empirical formulas but which differed in overall molecular weight, the larger of the compounds being described as "polymers" of the smallest. By this long-superseded, pre-structural definition, glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
(C6H12O6) was viewed as a polymer of formaldehyde
Formaldehyde ( , ) ( systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section ...
(CH2O).
New elements and gas laws
 English chemist
English chemist Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for ...
was a pioneer in the field of electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
, using Alessandro Volta's voltaic pile to split up common compounds and thus isolate a series of new elements. He went on to electrolyse molten salts and discovered several new metals, especially sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
and potassium
Potassium is the chemical element with the symbol K (from Neo-Latin '' kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmos ...
, highly reactive elements known as the alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s. Potassium, the first metal that was isolated by electrolysis, was discovered in 1807 by Davy, who derived it from caustic potash
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exploi ...
(KOH). Before the 19th century, no distinction was made between potassium and sodium. Sodium was first isolated by Davy in the same year by passing an electric current through molten sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and al ...
(NaOH). When Davy heard that Berzelius and Pontin prepared calcium amalgam by electrolyzing lime in mercury, he tried it himself. Davy was successful, and discovered calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar ...
in 1808 by electrolyzing a mixture of lime and mercuric oxide.magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
, strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is e ...
barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
.nitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or nos, is a chemical compound, an oxide of nitrogen with the formula . At room temperature, it is a colourless non-flammable gas, and ha ...
, which came to be known as laughing gas. Chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
was discovered in 1774 by Swedish chemist Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydr ...
, who called it ''"dephlogisticated marine acid"'' (see phlogiston theory
The phlogiston theory is a superseded scientific theory that postulated the existence of a fire-like element called phlogiston () contained within combustible bodies and released during combustion. The name comes from the Ancient Greek (''bur ...
) and mistakenly thought it contained oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
. Scheele observed several properties of chlorine gas, such as its bleaching effect on litmus, its deadly effect on insects, its yellow-green colour, and the similarity of its smell to that of aqua regia. However, Scheele was unable to publish his findings at the time. In 1810, chlorine was given its current name by Humphry Davy (derived from the Greek word for green), who insisted that chlorine was in fact an element. He also showed that oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
could not be obtained from the substance known as oxymuriatic acid (HCl solution). This discovery overturned Lavoisier's definition of acids as compounds of oxygen. Davy was a popular lecturer and able experimenter.
 French chemist Joseph Louis Gay-Lussac shared the interest of Lavoisier and others in the quantitative study of the properties of gases. From his first major program of research in 1801–1802, he concluded that equal volumes of all gases expand equally with the same increase in temperature: this conclusion is usually called " Charles's law", as Gay-Lussac gave credit to Jacques Charles, who had arrived at nearly the same conclusion in the 1780s but had not published it.
French chemist Joseph Louis Gay-Lussac shared the interest of Lavoisier and others in the quantitative study of the properties of gases. From his first major program of research in 1801–1802, he concluded that equal volumes of all gases expand equally with the same increase in temperature: this conclusion is usually called " Charles's law", as Gay-Lussac gave credit to Jacques Charles, who had arrived at nearly the same conclusion in the 1780s but had not published it.
English translation (extract).
br />
On page 157, Gay-Lussac mentions the unpublished findings of Charles: "''Avant d'aller plus loin, je dois prévenir que quoique j'eusse reconnu un grand nombre de fois que les gaz oxigène, azote, hydrogène et acide carbonique, et l'air atmosphérique se dilatent également depuis 0° jusqu'a 80°, le cit. Charles avait remarqué depuis 15 ans la même propriété dans ces gaz ; mais n'avant jamais publié ses résultats, c'est par le plus grand hasard que je les ai connus''." (Before going further, I should inform outhat although I had recognized many times that the gases oxygen, nitrogen, hydrogen, and carbonic acid .e., carbon dioxide and atmospheric air also expand from 0° to 80°, citizen Charles had noticed 15 years ago the same property in these gases; but having never published his results, it is by the merest chance that I knew of them.)École Polytechnique
École may refer to:
* an elementary school in the French educational stages normally followed by secondary education establishments (collège and lycée)
* École (river), a tributary of the Seine flowing in région Île-de-France
* École, Savoi ...
, Louis Jacques Thénard, Gay-Lussac also participated in early electrochemical research, investigating the elements discovered by its means. Among other achievements, they decomposed boric acid by using fused potassium, thus discovering the element boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has t ...
. The two also took part in contemporary debates that modified Lavoisier's definition of acids and furthered his program of analyzing organic compounds for their oxygen and hydrogen content.
The element iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
was discovered by French chemist Bernard Courtois in 1811.[ In French, seaweed that had been washed onto the shore was called "varec", "varech", or "vareck", whence the English word "wrack". Later, "varec" also referred to the ashes of such seaweed: The ashes were used as a source of iodine and salts of sodium and potassium.] Courtois gave samples to his friends, Charles Bernard Desormes
Charles Bernard Desormes (; 3 June 1777 – 30 August 1862) was a French physicist and chemist. He determined the ratio of the specific heats of gases in 1819. He did this and almost all his scientific work in collaboration with his son-in-law N ...
(1777–1862) and Nicolas Clément
Nicolas Clément (12 January 1779 – 21 November 1841) was a French physicist and chemist.
He was a colleague of Charles Desormes, with whom he conducted the Clément-Desormes experiment. The two chemists are also credited with determining a ...
(1779–1841), to continue research. He also gave some of the substance to Gay-Lussac and to physicist André-Marie Ampère
André-Marie Ampère (, ; ; 20 January 177510 June 1836) was a French physicist and mathematician who was one of the founders of the science of classical electromagnetism, which he referred to as "electrodynamics". He is also the inventor of nu ...
. On December 6, 1813, Gay-Lussac announced that the new substance was either an element or a compound of oxygen.Royal Society of London
The Royal Society, formally The Royal Society of London for Improving Natural Knowledge, is a learned society and the United Kingdom's national academy of sciences. The society fulfils a number of roles: promoting science and its benefits, r ...
stating that he had identified a new element. Arguments erupted between Davy and Gay-Lussac over who identified iodine first, but both scientists acknowledged Courtois as the first to isolate the element.
In 1815, Humphry Davy invented the Davy lamp, which allowed miners within coal mines
Coal mining is the process of extracting coal from the ground. Coal is valued for its energy content and since the 1880s has been widely used to generate electricity. Steel and cement industries use coal as a fuel for extraction of iron fro ...
to work safely in the presence of flammable gases. There had been many mining explosions caused by firedamp or methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane ...
often ignited by open flames of the lamps then used by miners. Davy conceived of using an iron gauze to enclose a lamp's flame, and so prevent the methane burning inside the lamp from passing out to the general atmosphere. Although the idea of the safety lamp had already been demonstrated by William Reid Clanny and by the then unknown (but later very famous) engineer George Stephenson, Davy's use of wire gauze to prevent the spread of flame was used by many other inventors in their later designs. There was some discussion as to whether Davy had discovered the principles behind his lamp without the help of the work of Smithson Tennant, but it was generally agreed that the work of both men had been independent. Davy refused to patent the lamp, and its invention led to him being awarded the Rumford medal in 1816.[David Knight, ‘Davy, Sir Humphry, baronet (1778–1829)’, ]Oxford Dictionary of National Biography
The ''Dictionary of National Biography'' (''DNB'') is a standard work of reference on notable figures from British history, published since 1885. The updated ''Oxford Dictionary of National Biography'' (''ODNB'') was published on 23 September ...
, Oxford University Press
Oxford University Press (OUP) is the university press of the University of Oxford. It is the largest university press in the world, and its printing history dates back to the 1480s. Having been officially granted the legal right to print book ...
, 200
accessed 6 April 2008
/ref>
 After Dalton published his atomic theory in 1808, certain of his central ideas were soon adopted by most chemists. However, uncertainty persisted for half a century about how atomic theory was to be configured and applied to concrete situations; chemists in different countries developed several different incompatible atomistic systems. A paper that suggested a way out of this difficult situation was published as early as 1811 by the Italian physicist Amedeo Avogadro (1776–1856), who hypothesized that equal volumes of gases at the same
After Dalton published his atomic theory in 1808, certain of his central ideas were soon adopted by most chemists. However, uncertainty persisted for half a century about how atomic theory was to be configured and applied to concrete situations; chemists in different countries developed several different incompatible atomistic systems. A paper that suggested a way out of this difficult situation was published as early as 1811 by the Italian physicist Amedeo Avogadro (1776–1856), who hypothesized that equal volumes of gases at the same temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
and pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country a ...
contain equal numbers of molecules, from which it followed that relative molecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
s of any two gases are the same as the ratio of the densities of the two gases under the same conditions of temperature and pressure. Avogadro also reasoned that simple gases were not formed of solitary atoms but were instead compound molecules of two or more atoms. Thus Avogadro was able to overcome the difficulty that Dalton and others had encountered when Gay-Lussac reported that above 100 °C the volume of water vapor was twice the volume of the oxygen used to form it. According to Avogadro, the molecule of oxygen had split into two atoms in the course of forming water vapor.
Avogadro's hypothesis was neglected for half a century after it was first published. Many reasons for this neglect have been cited, including some theoretical problems, such as Jöns Jacob Berzelius's "dualism", which asserted that compounds are held together by the attraction of positive and negative electrical charges, making it inconceivable that a molecule composed of two electrically similar atoms—as in oxygen—could exist. An additional barrier to acceptance was the fact that many chemists were reluctant to adopt physical methods (such as vapour-density determinations) to solve their problems. By mid-century, however, some leading figures had begun to view the chaotic multiplicity of competing systems of atomic weights and molecular formulas as intolerable. Moreover, purely chemical evidence began to mount that suggested Avogadro's approach might be right after all. During the 1850s, younger chemists, such as Alexander Williamson in England, Charles Gerhardt and Charles-Adolphe Wurtz
Charles Adolphe Wurtz (; 26 November 181710 May 1884) was an Alsatian French chemist. He is best remembered for his decades-long advocacy for the atomic theory and for ideas about the structures of chemical compounds, against the skeptical opinio ...
in France, and August Kekulé in Germany, began to advocate reforming theoretical chemistry to make it consistent with Avogadrian theory.
Wöhler and the vitalism debate
 In 1825,
In 1825, Friedrich Wöhler
Friedrich Wöhler () FRS(For) Hon FRSE (31 July 180023 September 1882) was a German chemist known for his work in inorganic chemistry, being the first to isolate the chemical elements beryllium and yttrium in pure metallic form. He was the fi ...
and Justus von Liebig
Justus Freiherr von Liebig (12 May 1803 – 20 April 1873) was a German scientist who made major contributions to agricultural and biological chemistry, and is considered one of the principal founders of organic chemistry. As a professor at th ...
performed the first confirmed discovery and explanation of isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Is ...
s, earlier named by Berzelius. Working with cyanic acid and fulminic acid, they correctly deduced that isomerism was caused by differing arrangements of atoms within a molecular structure. In 1827, William Prout classified biomolecules into their modern groupings: carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may o ...
s, protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s and lipid
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids in ...
s. After the nature of combustion was settled, a dispute about vitalism
Vitalism is a belief that starts from the premise that "living organisms are fundamentally different from non-living entities because they contain some non-physical element or are governed by different principles than are inanimate things." Wher ...
and the essential distinction between organic and inorganic substances began. The vitalism question was revolutionized in 1828 when Friedrich Wöhler synthesized urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
, thereby establishing that organic compounds could be produced from inorganic starting materials and disproving the theory of vitalism.
This opened a new research field in chemistry, and by the end of the 19th century, scientists were able to synthesize hundreds of organic compounds. The most important among them are mauve
Mauve (, ; , ) is a pale purple color named after the mallow flower (French: ''mauve''). The first use of the word ''mauve'' as a color was in 1796–98 according to the ''Oxford English Dictionary'', but its use seems to have been rare befo ...
, magenta, and other synthetic dye
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and ...
s, as well as the widely used drug aspirin
Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat inc ...
. The discovery of the artificial synthesis of urea contributed greatly to the theory of isomerism, as the empirical chemical formulas for urea and ammonium cyanate are identical (see Wöhler synthesis). In 1832, Friedrich Wöhler and Justus von Liebig discovered and explained functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the r ...
s and radicals
Radical may refer to:
Politics and ideology Politics
*Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe and ...
in relation to organic chemistry, as well as first synthesizing benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor. ...
. Liebig, a German chemist, made major contributions to agricultural
Agriculture or farming is the practice of cultivating plants and livestock. Agriculture was the key development in the rise of sedentary human civilization, whereby farming of domesticated species created food surpluses that enabled peopl ...
and biological chemistry, and worked on the organization of organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
. Liebig is considered the "father of the fertilizer
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from ...
industry" for his discovery of nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
as an essential plant nutrient
A nutrient is a substance used by an organism to survive, grow, and reproduce. The requirement for dietary nutrient intake applies to animals, plants, fungi, and protists. Nutrients can be incorporated into cells for metabolic purposes or excre ...
, and his formulation of the Law of the Minimum
Liebig's law of the minimum, often simply called Liebig's law or the law of the minimum, is a principle developed in agricultural science by Carl Sprengel (1840) and later popularized by Justus von Liebig. It states that growth is dictated not by t ...
which described the effect of individual nutrients on crops.
Mid-1800s
In 1840, Germain Hess proposed Hess's law, an early statement of the law of conservation of energy, which establishes that energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of ...
changes in a chemical process depend only on the states of the starting and product materials and not on the specific pathway taken between the two states. In 1847, Hermann Kolbe obtained acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
from completely inorganic sources, further disproving vitalism. In 1848, William Thomson, 1st Baron Kelvin (commonly known as Lord Kelvin) established the concept of absolute zero
Absolute zero is the lowest limit of the thermodynamic temperature scale, a state at which the enthalpy and entropy of a cooled ideal gas reach their minimum value, taken as zero kelvin. The fundamental particles of nature have minimum vibra ...
, the temperature at which all molecular motion ceases. In 1849, Louis Pasteur
Louis Pasteur (, ; 27 December 1822 – 28 September 1895) was a French chemist and microbiologist renowned for his discoveries of the principles of vaccination, microbial fermentation and pasteurization, the latter of which was named afte ...
discovered that the racemic form of tartaric acid
Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes, but also in bananas, tamarinds, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally ...
is a mixture of the levorotatory and dextrotatory forms, thus clarifying the nature of optical rotation and advancing the field of stereochemistry. In 1852, August Beer August Beer (; 31 July 1825 – 18 November 1863) was a German physicist, chemist, and mathematician of Jewish descent.
Biography
Beer was born in Trier, where he studied mathematics and natural sciences. Beer was educated at the technical s ...
proposed Beer's law, which explains the relationship between the composition of a mixture and the amount of light it will absorb. Based partly on earlier work by Pierre Bouguer and Johann Heinrich Lambert, it established the analytical technique known as spectrophotometry
Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. Spectrophotometry uses photometers, known as sp ...
. In 1855, Benjamin Silliman, Jr. pioneered methods of petroleum cracking, which made the entire modern petrochemical industry
The petrochemical industry is concerned with the production and trade of petrochemicals. A major part is constituted by the plastics (polymer) industry. It directly interfaces with the petroleum industry, especially the downstream sector.
Comp ...
possible.
 Avogadro's hypothesis began to gain broad appeal among chemists only after his compatriot and fellow scientist Stanislao Cannizzaro demonstrated its value in 1858, two years after Avogadro's death. Cannizzaro's chemical interests had originally centered on natural products and on reactions of
Avogadro's hypothesis began to gain broad appeal among chemists only after his compatriot and fellow scientist Stanislao Cannizzaro demonstrated its value in 1858, two years after Avogadro's death. Cannizzaro's chemical interests had originally centered on natural products and on reactions of aromatic compound
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past groupin ...
s; in 1853 he discovered that when benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor. ...
is treated with concentrated base, both benzoic acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin ...
and benzyl alcohol are produced—a phenomenon known today as the Cannizzaro reaction. In his 1858 pamphlet, Cannizzaro showed that a complete return to the ideas of Avogadro could be used to construct a consistent and robust theoretical structure that fit nearly all of the available empirical evidence. For instance, he pointed to evidence that suggested that not all elementary gases consist of two atoms per molecule—some were monatomic, most were diatomic
Diatomic molecules () are molecules composed of only two atoms, of the same or different chemical elements. If a diatomic molecule consists of two atoms of the same element, such as hydrogen () or oxygen (), then it is said to be homonuclear. O ...
, and a few were even more complex.
Another point of contention had been the formulas for compounds of the alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s (such as sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
) and the alkaline earth metals (such as calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar ...
), which, in view of their striking chemical analogies, most chemists had wanted to assign to the same formula type. Cannizzaro argued that placing these metals in different categories had the beneficial result of eliminating certain anomalies when using their physical properties to deduce atomic weights. Unfortunately, Cannizzaro's pamphlet was published initially only in Italian and had little immediate impact. The real breakthrough came with an international chemical congress held in the German town of Karlsruhe
Karlsruhe ( , , ; South Franconian: ''Kallsruh'') is the third-largest city of the German state (''Land'') of Baden-Württemberg after its capital of Stuttgart and Mannheim, and the 22nd-largest city in the nation, with 308,436 inhabitants. ...
in September 1860, at which most of the leading European chemists were present. The Karlsruhe Congress had been arranged by Kekulé, Wurtz, and a few others who shared Cannizzaro's sense of the direction chemistry should go. Speaking in French (as everyone there did), Cannizzaro's eloquence and logic made an indelible impression on the assembled body. Moreover, his friend Angelo Pavesi distributed Cannizzaro's pamphlet to attendees at the end of the meeting; more than one chemist later wrote of the decisive impression the reading of this document provided. For instance, Lothar Meyer later wrote that on reading Cannizzaro's paper, "The scales seemed to fall from my eyes." Cannizzaro thus played a crucial role in winning the battle for reform. The system advocated by him, and soon thereafter adopted by most leading chemists, is substantially identical to what is still used today.
Perkin, Crookes, and Nobel
In 1856, Sir William Henry Perkin
Sir William Henry Perkin (12 March 1838 – 14 July 1907) was a British chemist and entrepreneur best known for his serendipitous discovery of the first commercial synthetic organic dye, mauveine, made from aniline. Though he failed in trying ...
, age 18, given a challenge by his professor, August Wilhelm von Hofmann
August Wilhelm von Hofmann (8 April 18185 May 1892) was a German chemist who made considerable contributions to organic chemistry. His research on aniline helped lay the basis of the aniline-dye industry, and his research on coal tar laid the g ...
, sought to synthesize quinine
Quinine is a medication used to treat malaria and babesiosis. This includes the treatment of malaria due to '' Plasmodium falciparum'' that is resistant to chloroquine when artesunate is not available. While sometimes used for nocturnal leg ...
, the anti-malaria
Malaria is a mosquito-borne infectious disease that affects humans and other animals. Malaria causes symptoms that typically include fever, tiredness, vomiting, and headaches. In severe cases, it can cause jaundice, seizures, coma, or death. ...
drug, from coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat pso ...
. In one attempt, Perkin oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
aniline using potassium dichromate, whose toluidine impurities reacted with the aniline and yielded a black solid—suggesting a "failed" organic synthesis. Cleaning the flask with alcohol, Perkin noticed purple portions of the solution: a byproduct of the attempt was the first synthetic dye, known as mauveine
Mauveine, also known as aniline purple and Perkin's mauve, was one of the first synthetic dyes. It was discovered serendipitously by William Henry Perkin in 1856 while he was attempting to synthesise the phytochemical quinine for the treatment of ...
or Perkin's mauve. Perkin's discovery is the foundation of the dye synthesis industry, one of the earliest successful chemical industries.
German chemist August Kekulé von Stradonitz's most important single contribution was his structural theory of organic composition, outlined in two articles published in 1857 and 1858 and treated in great detail in the pages of his extraordinarily popular ''Lehrbuch der organischen Chemie'' ("Textbook of Organic Chemistry"), the first installment of which appeared in 1859 and gradually extended to four volumes. Kekulé argued that tetravalent carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
atoms – that is, carbon forming exactly four chemical bonds – could link together to form what he called a "carbon chain" or a "carbon skeleton," to which other atoms with other valences (such as hydrogen, oxygen, nitrogen, and chlorine) could join. He was convinced that it was possible for the chemist to specify this detailed molecular architecture for at least the simpler organic compounds known in his day. Kekulé was not the only chemist to make such claims in this era. The Scottish chemist Archibald Scott Couper
Archibald Scott Couper (; 31 March 1831 – 11 March 1892) was a Scottish chemist who proposed an early theory of chemical structure and bonding. He developed the concepts of tetravalent carbon atoms linking together to form large molecules ...
published a substantially similar theory nearly simultaneously, and the Russian chemist Aleksandr Butlerov did much to clarify and expand structure theory. However, it was predominantly Kekulé's ideas that prevailed in the chemical community.
 British chemist and physicist William Crookes is noted for his cathode ray studies, fundamental in the development of
British chemist and physicist William Crookes is noted for his cathode ray studies, fundamental in the development of atomic physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. Atomic physics typically refers to the study of atomic structure and the interaction between atoms. It is primarily concerned wit ...
. His researches on electrical discharges through a rarefied gas led him to observe the dark space around the cathode, now called the Crookes dark space. He demonstrated that cathode rays travel in straight lines and produce phosphorescence and heat when they strike certain materials. A pioneer of vacuum tubes, Crookes invented the Crookes tube – an early experimental discharge tube, with partial vacuum with which he studied the behavior of cathode rays. With the introduction of spectrum analysis by Robert Bunsen and Gustav Kirchhoff (1859–1860), Crookes applied the new technique to the study of selenium compounds. Bunsen and Kirchhoff had previously used spectroscopy as a means of chemical analysis to discover caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
and rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
. In 1861, Crookes used this process to discover thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes an ...
in some seleniferous deposits. He continued work on that new element, isolated it, studied its properties, and in 1873 determined its atomic weight. During his studies of thallium, Crookes discovered the principle of the Crookes radiometer, a device that converts light radiation into rotary motion. The principle of this radiometer has found numerous applications in the development of sensitive measuring instruments.
In 1862, Alexander Parkes exhibited Parkesine, one of the earliest synthetic polymers, at the International Exhibition in London. This discovery formed the foundation of the modern plastics industry The plastics industry manufactures polymer materials—commonly called plastics—and offers services in plastics important to a range of industries, including packaging, building and construction, electronics, aerospace, and transportation.
It is ...
. In 1864, Cato Maximilian Guldberg
Cato Maximilian Guldberg (11 August 1836 – 14 January 1902) was a Norwegian mathematician and chemist. Guldberg is best known as a pioneer in physical chemistry.
Background
Guldberg was born in Christiania (now Oslo), Norway. He was the el ...
and Peter Waage, building on Claude Louis Berthollet's ideas, proposed the law of mass action. In 1865, Johann Josef Loschmidt determined the number of molecules in a mole, later named Avogadro's number
The Avogadro constant, commonly denoted or , is the proportionality factor that relates the number of constituent particles (usually molecules, atoms or ions) in a sample with the amount of substance in that sample. It is an SI defining con ...
.
In 1865, August Kekulé, based partially on the work of Loschmidt and others, established the structure of benzene as a six carbon ring with alternating single and double bonds. Kekulé's novel proposal for benzene's cyclic structure was much contested but was never replaced by a superior theory. This theory provided the scientific basis for the dramatic expansion of the German chemical industry in the last third of the 19th century. Kekulé is also famous for having clarified the nature of aromatic compounds, which are compounds based on the benzene molecule. In 1865, Adolf von Baeyer
Johann Friedrich Wilhelm Adolf von Baeyer (; 31 October 1835 – 20 August 1917) was a German chemist who synthesised indigo and developed a nomenclature for cyclic compounds (that was subsequently extended and adopted as part of the IUPAC org ...
began work on indigo dye
Indigo dye is an organic compound with a distinctive blue color. Historically, indigo was a natural dye extracted from the leaves of some plants of the ''Indigofera'' genus, in particular '' Indigofera tinctoria''; dye-bearing ''Indigofera'' pl ...
, a milestone in modern industrial organic chemistry which revolutionized the dye industry.
Swedish chemist and inventor Alfred Nobel found that when nitroglycerin
Nitroglycerin (NG), (alternative spelling of nitroglycerine) also known as trinitroglycerin (TNG), nitro, glyceryl trinitrate (GTN), or 1,2,3-trinitroxypropane, is a dense, colorless, oily, explosive liquid most commonly produced by nitrating g ...
was incorporated in an absorbent inert substance like ''kieselguhr'' (diatomaceous earth
Diatomaceous earth (), diatomite (), or kieselgur/kieselguhr is a naturally occurring, soft, siliceous sedimentary rock that can be crumbled into a fine white to off-white powder. It has a particle size ranging from more than 3 μm to l ...
) it became safer and more convenient to handle, and this mixture he patented in 1867 as dynamite
Dynamite is an explosive made of nitroglycerin, sorbents (such as powdered shells or clay), and stabilizers. It was invented by the Swedish chemist and engineer Alfred Nobel in Geesthacht, Northern Germany, and patented in 1867. It rapidl ...
. Nobel later on combined nitroglycerin with various nitrocellulose compounds, similar to collodion, but settled on a more efficient recipe combining another nitrate explosive, and obtained a transparent, jelly-like substance, which was a more powerful explosive than dynamite. Gelignite
Gelignite (), also known as blasting gelatin or simply "jelly", is an explosive material consisting of collodion- cotton (a type of nitrocellulose or guncotton) dissolved in either nitroglycerine or nitroglycol and mixed with wood pulp and saltp ...
, or blasting gelatin, as it was named, was patented in 1876; and was followed by a host of similar combinations, modified by the addition of potassium nitrate
Potassium nitrate is a chemical compound with the chemical formula . This alkali metal nitrate salt is also known as Indian saltpetre (large deposits of which were historically mined in India). It is an ionic salt of potassium ions K+ and ...
and various other substances.
Mendeleev's periodic table
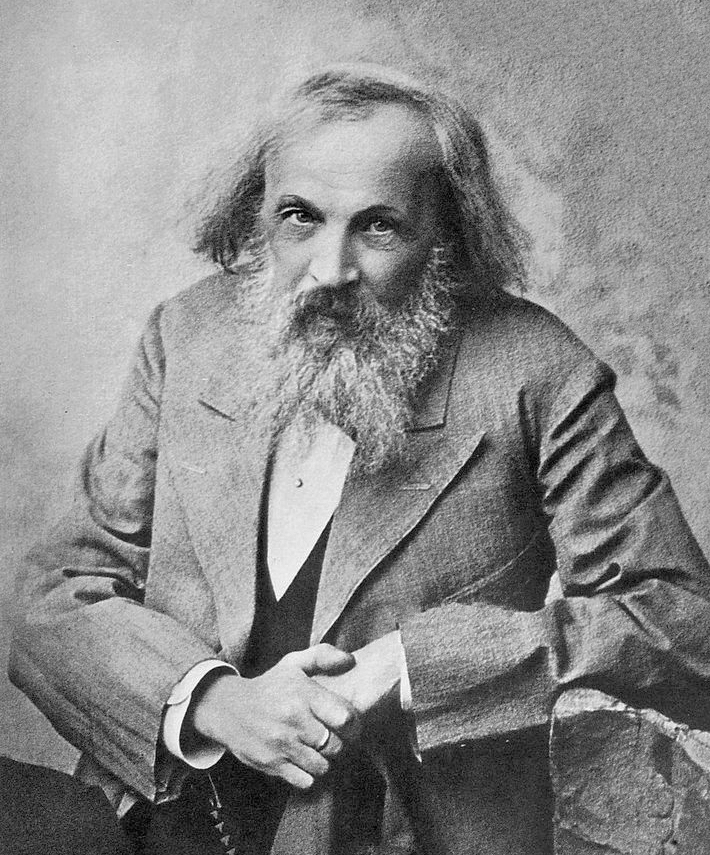 An important breakthrough in making sense of the list of known chemical elements (as well as in understanding the internal structure of atoms) was
An important breakthrough in making sense of the list of known chemical elements (as well as in understanding the internal structure of atoms) was Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
's development of the first modern periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, or the periodic classification of the elements. Mendeleev, a Russian chemist, felt that there was some type of order to the elements and he spent more than thirteen years of his life collecting data and assembling the concept, initially with the idea of resolving some of the disorder in the field for his students. Mendeleev found that, when all the known chemical elements were arranged in order of increasing atomic weight, the resulting table displayed a recurring pattern, or periodicity, of properties within groups of elements. Mendeleev's law allowed him to build up a systematic periodic table of all the 66 elements then known based on atomic mass, which he published in ''Principles of Chemistry'' in 1869. His first Periodic Table was compiled on the basis of arranging the elements in ascending order of atomic weight and grouping them by similarity of properties.
Mendeleev had such faith in the validity of the periodic law that he proposed changes to the generally accepted values for the atomic weight of a few elements and, in his version of the periodic table of 1871, predicted the locations within the table of unknown elements together with their properties. He even predicted the likely properties of three yet-to-be-discovered elements, which he called ekaboron (Eb), ekaaluminium (Ea), and ekasilicon (Es), which proved to be good predictors of the properties of scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in ...
, gallium
Gallium is a chemical element with the Symbol (chemistry), symbol Ga and atomic number 31. Discovered by France, French chemist Paul-Émile Lecoq de Boisbaudran in 1875, Gallium is in boron group, group 13 of the periodic table and is similar to ...
, and germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors ...
, respectively, which each fill the spot in the periodic table assigned by Mendeleev.
At first the periodic system did not raise interest among chemists. However, with the discovery of the predicted elements, notably gallium in 1875, scandium in 1879, and germanium in 1886, it began to win wide acceptance. The subsequent proof of many of his predictions within his lifetime brought fame to Mendeleev as the founder of the periodic law. This organization surpassed earlier attempts at classification by Alexandre-Émile Béguyer de Chancourtois
Alexandre-Émile Béguyer de Chancourtois (20 January 1820 – 14 November 1886) was a French geologist and mineralogist who was the first to arrange the chemical elements in order of atomic weights, doing so in 1862. De Chancourtois only publish ...
, who published the telluric helix, an early, three-dimensional version of the periodic table of the elements in 1862, John Newlands, who proposed the law of octaves (a precursor to the periodic law) in 1864, and Lothar Meyer, who developed an early version of the periodic table with 28 elements organized by valence in 1864. Mendeleev's table did not include any of the noble gases, however, which had not yet been discovered. Gradually the periodic law and table became the framework for a great part of chemical theory. By the time Mendeleev died in 1907, he enjoyed international recognition and had received distinctions and awards from many countries.
In 1873, Jacobus Henricus van 't Hoff and Joseph Achille Le Bel, working independently, developed a model of chemical bonding that explained the chirality experiments of Pasteur and provided a physical cause for optical activity
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circul ...
in chiral compounds. van 't Hoff's publication, called V''oorstel tot Uitbreiding der Tegenwoordige in de Scheikunde gebruikte Structuurformules in de Ruimte'', etc. (Proposal for the development of 3-dimensional chemical structural formulae) and consisting of twelve pages of text and one page of diagrams, gave the impetus to the development of stereochemistry. The concept of the "asymmetrical carbon atom", dealt with in this publication, supplied an explanation of the occurrence of numerous isomers, inexplicable by means of the then current structural formulae. At the same time he pointed out the existence of relationship between optical activity and the presence of an asymmetrical carbon atom.
Josiah Willard Gibbs
 American mathematical physicist J. Willard Gibbs's work on the applications of
American mathematical physicist J. Willard Gibbs's work on the applications of thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws ...
was instrumental in transforming physical chemistry
Physical chemistry is the study of macroscopic and microscopic phenomena in chemical systems in terms of the principles, practices, and concepts of physics such as motion, energy, force, time, thermodynamics, quantum chemistry, statistica ...
into a rigorous deductive science. During the years from 1876 to 1878, Gibbs worked on the principles of thermodynamics, applying them to the complex processes involved in chemical reactions. He discovered the concept of chemical potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a speci ...
, or the "fuel" that makes chemical reactions work. In 1876 he published his most famous contribution, "On the Equilibrium of Heterogeneous Substances
In the history of thermodynamics, ''On the Equilibrium of Heterogeneous Substances'' is a 300-page paper written by American chemical physicist Willard Gibbs. It is one of the founding papers in thermodynamics, along with German physicist Hermann ...
", a compilation of his work on thermodynamics and physical chemistry which laid out the concept of free energy to explain the physical basis of chemical equilibria. In these essays were the beginnings of Gibbs’ theories of phases of matter: he considered each state of matter a phase, and each substance a component. Gibbs took all of the variables involved in a chemical reaction – temperature, pressure, energy, volume, and entropy – and included them in one simple equation known as Gibbs' phase rule.
Within this paper was perhaps his most outstanding contribution, the introduction of the concept of free energy, now universally called Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature an ...
in his honor. The Gibbs free energy relates the tendency of a physical or chemical system to simultaneously lower its energy and increase its disorder, or entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodyna ...
, in a spontaneous natural process. Gibbs's approach allows a researcher to calculate the change in free energy in the process, such as in a chemical reaction, and how fast it will happen. Since virtually all chemical processes and many physical ones involve such changes, his work has significantly impacted both the theoretical and experiential aspects of these sciences. In 1877, Ludwig Boltzmann established statistical derivations of many important physical and chemical concepts, including entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodyna ...
, and distributions of molecular velocities in the gas phase. Together with Boltzmann and James Clerk Maxwell
James Clerk Maxwell (13 June 1831 – 5 November 1879) was a Scottish mathematician and scientist responsible for the classical theory of electromagnetic radiation, which was the first theory to describe electricity, magnetism and ligh ...
, Gibbs created a new branch of theoretical physics called statistical mechanics (a term that he coined), explaining the laws of thermodynamics as consequences of the statistical properties of large ensembles of particles. Gibbs also worked on the application of Maxwell's equations to problems in physical optics. Gibbs's derivation of the phenomenological laws of thermodynamics from the statistical properties of systems with many particles was presented in his highly influential textbook '' Elementary Principles in Statistical Mechanics'', published in 1902, a year before his death. In that work, Gibbs reviewed the relationship between the laws of thermodynamics and the statistical theory of molecular motions. The overshooting of the original function by partial sums of Fourier series
A Fourier series () is a summation of harmonically related sinusoidal functions, also known as components or harmonics. The result of the summation is a periodic function whose functional form is determined by the choices of cycle length (or '' ...
at points of discontinuity is known as the Gibbs phenomenon.
Late 19th century
German engineer Carl von Linde's invention of a continuous process of liquefying gases in large quantities formed a basis for the modern technology of refrigeration and provided both impetus and means for conducting scientific research at low temperatures and very high vacuums. He developed a dimethyl ether
Dimethyl ether (DME; also known as methoxymethane) is the organic compound with the formula CH3OCH3,
(sometimes ambiguously simplified to C2H6O as it is an isomer of ethanol). The simplest ether, it is a colorless gas that is a useful precursor ...
refrigerator (1874) and an ammonia refrigerator (1876). Though other refrigeration units had been developed earlier, Linde's were the first to be designed with the aim of precise calculations of efficiency. In 1895 he set up a large-scale plant for the production of liquid air. Six years later he developed a method for separating pure liquid oxygen from liquid air that resulted in widespread industrial conversion to processes utilizing oxygen (e.g., in steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistan ...
manufacture).
In 1883, Svante Arrhenius developed an ion theory to explain conductivity in electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon ...
s. In 1884, Jacobus Henricus van 't Hoff published ''Études de Dynamique chimique'' (Studies in Dynamic Chemistry), a seminal study on chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in ...
. In this work, van 't Hoff entered for the first time the field of physical chemistry. Of great importance was his development of the general thermodynamic relationship between the heat of conversion and the displacement of the equilibrium as a result of temperature variation. At constant volume, the equilibrium in a system will tend to shift in such a direction as to oppose the temperature change which is imposed upon the system. Thus, lowering the temperature results in heat development while increasing the temperature results in heat absorption. This principle of mobile equilibrium was subsequently (1885) put in a general form by Henry Louis Le Chatelier, who extended the principle to include compensation, by change of volume, for imposed pressure changes. The van 't Hoff-Le Chatelier principle, or simply Le Chatelier's principle
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French ...
, explains the response of dynamic
Dynamics (from Greek δυναμικός ''dynamikos'' "powerful", from δύναμις ''dynamis'' "power") or dynamic may refer to:
Physics and engineering
* Dynamics (mechanics)
** Aerodynamics, the study of the motion of air
** Analytical dyn ...
chemical equilibria to external stresses.
In 1884, Hermann Emil Fischer
Hermann Emil Louis Fischer (; 9 October 1852 – 15 July 1919) was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He also developed the Fischer projection, a symbolic way of draw ...
proposed the structure of purine
Purine is a heterocyclic aromatic organic compound that consists of two rings ( pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines ...
, a key structure in many biomolecules, which he later synthesized in 1898. He also began work on the chemistry of glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
and related sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or do ...
s. In 1885, Eugen Goldstein named the cathode ray, later discovered to be composed of electrons, and the canal ray, later discovered to be positive hydrogen ions that had been stripped of their electrons in a cathode ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms ( oscilloscope), ...
; these would later be named proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s. The year 1885 also saw the publishing of J. H. van 't Hoff's ''L'Équilibre chimique dans les Systèmes gazeux ou dissous à I'État dilué'' (Chemical equilibria in gaseous systems or strongly diluted solutions), which dealt with this theory of dilute solutions. Here he demonstrated that the "osmotic pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane.
It is also defined as the measure of the tendency of a solution to take in a pure ...
" in solutions which are sufficiently dilute is proportionate to the concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', ''number concentration'', ...
and the absolute temperature so that this pressure can be represented by a formula that only deviates from the formula for gas pressure by a coefficient i. He also determined the value of ''i'' by various methods, for example by means of the vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed pha ...
and François-Marie Raoult's results on the lowering of the freezing point. Thus van 't Hoff was able to prove that thermodynamic laws are not only valid for gases, but also for dilute solutions. His pressure laws, given general validity by the electrolytic dissociation theory of Arrhenius (1884–1887) – the first foreigner who came to work with him in Amsterdam (1888) – are considered the most comprehensive and important in the realm of natural sciences. In 1893, Alfred Werner
Alfred Werner (12 December 1866 – 15 November 1919) was a Swiss chemist who was a student at ETH Zurich and a professor at the University of Zurich. He won the Nobel Prize in Chemistry in 1913 for proposing the octahedral configuration ...
discovered the octahedral structure of cobalt complexes, thus establishing the field of coordination chemistry
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Man ...
.
Ramsay's discovery of the noble gases
The most celebrated discoveries of Scottish chemist William Ramsay were made in inorganic chemistry. Ramsay was intrigued by the British physicist John Strutt, 3rd Baron Rayleigh's 1892 discovery that the atomic weight of nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
found in chemical compounds was lower than that of nitrogen found in the atmosphere. He ascribed this discrepancy to a light gas included in chemical compounds of nitrogen, while Ramsay suspected a hitherto undiscovered heavy gas in atmospheric nitrogen. Using two different methods to remove all known gases from air, Ramsay and Lord Rayleigh were able to announce in 1894 that they had found a monatomic, chemically inert gaseous element that constituted nearly 1 percent of the atmosphere; they named it argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice a ...
.
 The following year, Ramsay liberated another inert gas from a mineral called
The following year, Ramsay liberated another inert gas from a mineral called cleveite
Cleveite is an impure radioactive variety of uraninite containing uranium, found in Norway. It has the composition UO2 with about 10% of the uranium substituted by rare-earth elements. It was named after Swedish chemist Per Teodor Cleve.
Cleveit ...
; this proved to be helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic ta ...
, previously known only in the solar spectrum. In his book ''The Gases of the Atmosphere'' (1896), Ramsay showed that the positions of helium and argon in the periodic table of elements indicated that at least three more noble gases might exist. In 1898 Ramsay and the British chemist Morris W. Travers
Morris William Travers, FRS (24 January 1872 – 25 August 1961) was an English chemist who worked with Sir William Ramsay in the discovery of xenon, neon and krypton. His work on several of the rare gases earned him the name ''Rare gas ...
isolated these elements—called neon, krypton
Krypton (from grc, κρυπτός, translit=kryptos 'the hidden one') is a chemical element with the symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is of ...
, and xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
—from air and brought them to a liquid state at low temperature and high pressure. Sir William Ramsay worked with Frederick Soddy to demonstrate, in 1903, that alpha particles (helium nuclei) were continually produced during the radioactive decay of a sample of radium. Ramsay was awarded the 1904 Nobel Prize for Chemistry in recognition of "services in the discovery of the inert gaseous elements in the air, and his determination of their place in the periodic system."
In 1897, J. J. Thomson discovered the electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
using the cathode ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms ( oscilloscope), ...
. In 1898, Wilhelm Wien
Wilhelm Carl Werner Otto Fritz Franz Wien (; 13 January 1864 – 30 August 1928) was a German physicist who, in 1893, used theories about heat and electromagnetism to deduce Wien's displacement law, which calculates the emission of a blackbod ...
demonstrated that canal rays (streams of positive ions) can be deflected by magnetic fields and that the amount of deflection is proportional to the mass-to-charge ratio. This discovery would lead to the analytical technique known as mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
in 1912.
Marie and Pierre Curie

Marie Skłodowska-Curie
Marie Salomea Skłodowska–Curie ( , , ; born Maria Salomea Skłodowska, ; 7 November 1867 – 4 July 1934) was a Polish and naturalized-French physicist and chemist who conducted pioneering research on radioactivity. She was the first ...
was a Polish-born French physicist and chemist who is famous for her pioneering research on radioactivity. She and her husband are considered to have laid the cornerstone of the nuclear age with their research on radioactivity. Marie was fascinated with the work of Henri Becquerel, a French physicist who discovered in 1896 that uranium casts off rays similar to the X-ray
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 picometers to 10 nanometers, corresponding to frequencies in the range 30&nb ...
s discovered by Wilhelm Röntgen
Wilhelm Conrad Röntgen (; ; 27 March 184510 February 1923) was a German mechanical engineer and physicist, who, on 8 November 1895, produced and detected electromagnetic radiation in a wavelength range known as X-rays or Röntgen rays, an achie ...
. Marie Curie began studying uranium in late 1897 and theorized, according to a 1904 article she wrote for Century magazine, "that the emission of rays by the compounds of uranium is a property of the metal itself—that it is an atomic property of the element uranium independent of its chemical or physical state." Curie took Becquerel's work a few steps further, conducting her own experiments on uranium rays. She discovered that the rays remained constant, no matter the condition or form of the uranium. The rays, she theorized, came from the element's atomic structure. This revolutionary idea created the field of atomic physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. Atomic physics typically refers to the study of atomic structure and the interaction between atoms. It is primarily concerned wit ...
and the Curies coined the word ''radioactivity'' to describe the phenomena.
 Pierre and Marie further explored radioactivity by working to separate the substances in uranium ores and then using the electrometer to make radiation measurements to ‘trace’ the minute amount of unknown radioactive element among the fractions that resulted. Working with the mineral pitchblende, the pair discovered a new radioactive element in 1898. They named the element polonium, after Marie's native country of Poland. On December 21, 1898, the Curies detected the presence of another radioactive material in the pitchblende. They presented this finding to the
Pierre and Marie further explored radioactivity by working to separate the substances in uranium ores and then using the electrometer to make radiation measurements to ‘trace’ the minute amount of unknown radioactive element among the fractions that resulted. Working with the mineral pitchblende, the pair discovered a new radioactive element in 1898. They named the element polonium, after Marie's native country of Poland. On December 21, 1898, the Curies detected the presence of another radioactive material in the pitchblende. They presented this finding to the French Academy of Sciences
The French Academy of Sciences (French: ''Académie des sciences'') is a learned society, founded in 1666 by Louis XIV at the suggestion of Jean-Baptiste Colbert, to encourage and protect the spirit of French scientific research. It was at ...
on December 26, proposing that the new element be called radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rat ...
. The Curies then went to work isolating polonium and radium from naturally occurring compounds to prove that they were new elements. In 1902, the Curies announced that they had produced a decigram of pure radium, demonstrating its existence as a unique chemical element. While it took three years for them to isolate radium, they were never able to isolate polonium. Along with the discovery of two new elements and finding techniques for isolating radioactive isotopes, Curie oversaw the world's first studies into the treatment of neoplasm
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
s, using radioactive isotopes. With Henri Becquerel and her husband, Pierre Curie, she was awarded the 1903 Nobel Prize for Physics. She was the sole winner of the 1911 Nobel Prize for Chemistry. She was the first woman to win a Nobel Prize, and she is the only woman to win the award in two different fields.
While working with Marie to extract pure substances from ores, an undertaking that really required industrial resources but that they achieved in relatively primitive conditions, Pierre himself concentrated on the physical study (including luminous and chemical effects) of the new radiations. Through the action of magnetic fields on the rays given out by the radium, he proved the existence of particles that were electrically positive, negative, and neutral; these Ernest Rutherford was afterward to call alpha, beta, and gamma rays. Pierre then studied these radiations by calorimetry
In chemistry and thermodynamics, calorimetry () is the science or act of measuring changes in ''state variables'' of a body for the purpose of deriving the heat transfer associated with changes of its state due, for example, to chemical re ...
and also observed the physiological effects of radium, thus opening the way to radium therapy. Among Pierre Curie's discoveries were that ferromagnetic substances exhibited a critical temperature transition, above which the substances lost their ferromagnetic behavior – this is known as the " Curie point." He was elected to the Academy of Sciences (1905), having in 1903 jointly with Marie received the Royal Society's prestigious Davy Medal and jointly with her and Becquerel the Nobel Prize for Physics. He was run over by a carriage in the rue Dauphine in Paris in 1906 and died instantly. His complete works were published in 1908.
Ernest Rutherford
 New Zealand-born chemist and physicist Ernest Rutherford is considered to be "the father of
New Zealand-born chemist and physicist Ernest Rutherford is considered to be "the father of nuclear physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies t ...
." Rutherford is best known for devising the names alpha
Alpha (uppercase , lowercase ; grc, ἄλφα, ''álpha'', or ell, άλφα, álfa) is the first letter of the Greek alphabet. In the system of Greek numerals, it has a value of one. Alpha is derived from the Phoenician letter aleph , whi ...
, beta
Beta (, ; uppercase , lowercase , or cursive ; grc, βῆτα, bē̂ta or ell, βήτα, víta) is the second letter of the Greek alphabet. In the system of Greek numerals, it has a value of 2. In Modern Greek, it represents the voiced labiod ...
, and gamma
Gamma (uppercase , lowercase ; ''gámma'') is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. In Ancient Greek, the letter gamma represented a voiced velar stop . In Modern Greek, this letter r ...
to classify various forms of radioactive "rays" which were poorly understood at his time (alpha and beta rays are particle beams, while gamma rays are a form of high-energy electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visib ...
). Rutherford deflected alpha rays with both electric and magnetic fields in 1903. Working with Frederick Soddy, Rutherford explained that radioactivity is due to the transmutation of elements, now known to involve nuclear reactions.
 He also observed that the intensity of radioactivity of a radioactive element decreases over a unique and regular amount of time until a point of stability, and he named the halving time the "
He also observed that the intensity of radioactivity of a radioactive element decreases over a unique and regular amount of time until a point of stability, and he named the halving time the "half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
." In 1901 and 1902 he worked with Frederick Soddy to prove that atoms of one radioactive element would spontaneously turn into another, by expelling a piece of the atom at high velocity. In 1906 at the University of Manchester, Rutherford oversaw an experiment conducted by his students Hans Geiger (known for the Geiger counter
A Geiger counter (also known as a Geiger–Müller counter) is an electronic instrument used for detecting and measuring ionizing radiation. It is widely used in applications such as radiation dosimetry, radiological protection, experimental p ...
) and Ernest Marsden. In the Geiger–Marsden experiment, a beam of alpha particles, generated by the radioactive decay of radon
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains th ...
, was directed normally onto a sheet of very thin gold foil in an evacuated chamber. Under the prevailing plum pudding model
The plum pudding model is one of several historical scientific models of the atom. First proposed by J. J. Thomson in 1904 soon after the discovery of the electron, but before the discovery of the atomic nucleus, the model tried to explain two ...
, the alpha particles should all have passed through the foil and hit the detector screen, or have been deflected by, at most, a few degrees.
However, the actual results surprised Rutherford. Although many of the alpha particles did pass through as expected, many others were deflected at small angles while others were reflected back to the alpha source. They observed that a very small percentage of particles were deflected through angles much larger than 90 degrees. The gold foil experiment showed large deflections for a small fraction of incident particles. Rutherford realized that, because some of the alpha particles were deflected or reflected, the atom had a concentrated centre of positive charge and of relatively large mass – Rutherford later termed this positive center the "atomic nucleus
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron ...
". The alpha particles had either hit the positive centre directly or passed by it close enough to be affected by its positive charge. Since many other particles passed through the gold foil, the positive centre would have to be a relatively small size compared to the rest of the atom – meaning that the atom is mostly open space. From his results, Rutherford developed a model of the atom that was similar to the solar system, known as the Rutherford model. Like planets, electrons orbited a central, sun-like nucleus. For his work with radiation and the atomic nucleus, Rutherford received the 1908 Nobel Prize in Chemistry.
20th century
 In 1903, Mikhail Tsvet invented chromatography, an important analytic technique. In 1904, Hantaro Nagaoka proposed an early nuclear model of the atom, where electrons orbit a dense massive nucleus. In 1905, Fritz Haber and Carl Bosch developed the Haber process for making
In 1903, Mikhail Tsvet invented chromatography, an important analytic technique. In 1904, Hantaro Nagaoka proposed an early nuclear model of the atom, where electrons orbit a dense massive nucleus. In 1905, Fritz Haber and Carl Bosch developed the Haber process for making ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
, a milestone in industrial chemistry with deep consequences in agriculture. The Haber process, or Haber-Bosch process, combined nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
to form ammonia in industrial quantities for the production of fertilizer and munitions. The food production for half the world's current population depends on this method for producing fertilizer. Haber, along with Max Born
Max Born (; 11 December 1882 – 5 January 1970) was a German physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a ...
, proposed the Born–Haber cycle
The Born–Haber cycle is an approach to analyze reaction energies. It was named after the two German scientists Max Born and Fritz Haber, who developed it in 1919. It was also independently formulated by Kasimir Fajans and published concurrently ...
as a method for evaluating the lattice energy of an ionic solid. Haber has also been described as the "father of chemical warfare
Chemical warfare (CW) involves using the toxic properties of chemical substances as weapons. This type of warfare is distinct from nuclear warfare, biological warfare and radiological warfare, which together make up CBRN, the military a ...
" for his work developing and deploying chlorine and other poisonous gases during World War I.
 In 1905,
In 1905, Albert Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theor ...
explained Brownian motion in a way that definitively proved atomic theory. Leo Baekeland invented bakelite
Polyoxybenzylmethylenglycolanhydride, better known as Bakelite ( ), is a thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde. The first plastic made from synthetic components, it was developed ...
, one of the first commercially successful plastics. In 1909, American physicist Robert Andrews Millikan
Robert Andrews Millikan (March 22, 1868 – December 19, 1953) was an American experimental physicist honored with the Nobel Prize for Physics in 1923 for the measurement of the elementary electric charge and for his work on the photoelectric ...
– who had studied in Europe under Walther Nernst and Max Planck – measured the charge of individual electrons with unprecedented accuracy through the oil drop experiment, in which he measured the electric charges on tiny falling water (and later oil) droplets. His study established that any particular droplet's electrical charge is a multiple of a definite, fundamental value — the electron's charge — and thus a confirmation that all electrons have the same charge and mass. Beginning in 1912, he spent several years investigating and finally proving Albert Einstein's proposed linear relationship between energy and frequency, and providing the first direct photoelectric support for Planck's constant. In 1923 Millikan was awarded the Nobel Prize for Physics.
In 1909, S. P. L. Sørensen invented the pH concept and developed methods for measuring acidity. In 1911, Antonius Van den Broek
Antonius Johannes van den Broek (4 May 1870, Zoetermeer – 25 October 1926, Bilthoven) was a Dutch amateur physicist notable for being the first who realized that the number of an element in the periodic table (now called atomic number) correspond ...
proposed the idea that the elements on the periodic table are more properly organized by positive nuclear charge rather than atomic weight. In 1911, the first Solvay Conference
The Solvay Conferences (french: Conseils Solvay) have been devoted to outstanding preeminent open problems in both physics and chemistry. They began with the historic invitation-only 1911 Solvay Conference on Physics, considered a turning point i ...
was held in Brussels, bringing together most of the most prominent scientists of the day. In 1912, William Henry Bragg and William Lawrence Bragg proposed Bragg's law and established the field of X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
, an important tool for elucidating the crystal structure of substances. In 1912, Peter Debye
Peter Joseph William Debye (; ; March 24, 1884 – November 2, 1966) was a Dutch-American physicist and physical chemist, and Nobel laureate in Chemistry.
Biography
Early life
Born Petrus Josephus Wilhelmus Debije in Maastricht, Netherland ...
used the concept of a molecular dipole to describe asymmetric charge distribution in some molecules.
Niels Bohr
 In 1913, Niels Bohr, a Danish physicist, introduced the concepts of
In 1913, Niels Bohr, a Danish physicist, introduced the concepts of quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, ...
to atomic structure by proposing what is now known as the Bohr model of the atom, where electrons exist only in strictly defined circular orbits around the nucleus similar to rungs on a ladder. The Bohr Model is a planetary model in which the negatively charged electrons orbit a small, positively charged nucleus similar to the planets orbiting the Sun (except that the orbits are not planar) – the gravitational force of the solar system is mathematically akin to the attractive Coulomb (electrical) force between the positively charged nucleus and the negatively charged electrons.
In the Bohr model, however, electrons orbit the nucleus in orbits that have a set size and energy – the energy levels are said to be ''quantized'', which means that only certain orbits with certain radii are allowed; orbits in between simply don't exist. The energy of the orbit is related to its size – that is, the lowest energy is found in the smallest orbit. Bohr also postulated that electromagnetic radiation is absorbed or emitted when an electron moves from one orbit to another. Because only certain electron orbits are permitted, the emission of light accompanying a jump of an electron from an excited energy state to ground state produces a unique emission spectrum
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a transition from a high energy state to a lower energy state. The photon energy of ...
for each element. Bohr later received the Nobel Prize in physics for this work.
Niels Bohr also worked on the principle of complementarity, which states that an electron can be interpreted in two mutually exclusive and valid ways. Electrons can be interpreted as wave or particle models. His hypothesis was that an incoming particle would strike the nucleus and create an excited compound nucleus. This formed the basis of his liquid drop model and later provided a theory base for nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
after its discovery
Discovery may refer to:
* Discovery (observation), observing or finding something unknown
* Discovery (fiction), a character's learning something unknown
* Discovery (law), a process in courts of law relating to evidence
Discovery, The Discove ...
by chemists Otto Hahn and Fritz Strassman
Friedrich Wilhelm Strassmann (; 22 February 1902 – 22 April 1980) was a German chemist who, with Otto Hahn in December 1938, identified the element barium as a product of the bombardment of uranium with neutrons. Their observation was the key ...
, and explanation and naming by physicists Lise Meitner
Elise Meitner ( , ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish physicist who was one of those responsible for the discovery of the element protactinium and nuclear fission. While working at the Kaiser Wilhelm Institute on r ...
and Otto Frisch
Otto Robert Frisch FRS (1 October 1904 – 22 September 1979) was an Austrian-born British physicist who worked on nuclear physics. With Lise Meitner he advanced the first theoretical explanation of nuclear fission (coining the term) and first ...
.
 In 1913, Henry Moseley, working from Van den Broek's earlier idea, introduced the concept of atomic number to fix some inadequacies of Mendeleev's periodic table, which had been based on atomic weight. The peak of Frederick Soddy's career in radiochemistry was in 1913 with his formulation of the concept of
In 1913, Henry Moseley, working from Van den Broek's earlier idea, introduced the concept of atomic number to fix some inadequacies of Mendeleev's periodic table, which had been based on atomic weight. The peak of Frederick Soddy's career in radiochemistry was in 1913 with his formulation of the concept of isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
s, which stated that certain elements exist in two or more forms which have different atomic weights but which are indistinguishable chemically. He is remembered for proving the existence of isotopes of certain radioactive elements, and is also credited, along with others, with the discovery of the element protactinium in 1917. In 1913, J. J. Thomson expanded on the work of Wien by showing that charged subatomic particles can be separated by their mass-to-charge ratio, a technique known as mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
.
Gilbert N. Lewis
American physical chemist Gilbert N. Lewis
Gilbert Newton Lewis (October 23 or October 25, 1875 – March 23, 1946) was an American physical chemist and a Dean of the College of Chemistry at University of California, Berkeley. Lewis was best known for his discovery of the covalent bond a ...
laid the foundation of valence bond theory; he was instrumental in developing a bonding theory based on the number of electrons in the outermost "valence" shell of the atom. In 1902, while Lewis was trying to explain valence to his students, he depicted atoms as constructed of a concentric series of cubes with electrons at each corner. This "cubic atom" explained the eight groups in the periodic table and represented his idea that chemical bonds are formed by electron transference to give each atom a complete set of eight outer electrons (an "octet").
Lewis's theory of chemical bonding continued to evolve and, in 1916, he published his seminal article "The Atom of the Molecule", which suggested that a chemical bond is a pair of electrons shared by two atoms. Lewis's model equated the classical chemical bond with the sharing of a pair of electrons between the two bonded atoms. Lewis introduced the "electron dot diagrams" in this paper to symbolize the electronic structures of atoms and molecules. Now known as Lewis structures
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons th ...
, they are discussed in virtually every introductory chemistry book.
Shortly after the publication of his 1916 paper, Lewis became involved with military research. He did not return to the subject of chemical bonding until 1923, when he masterfully summarized his model in a short monograph entitled Valence and the Structure of Atoms and Molecules. His renewal of interest in this subject was largely stimulated by the activities of the American chemist and General Electric researcher Irving Langmuir
Irving Langmuir (; January 31, 1881 – August 16, 1957) was an American chemist, physicist, and engineer. He was awarded the Nobel Prize in Chemistry in 1932 for his work in surface chemistry.
Langmuir's most famous publication is the 1919 ar ...
, who between 1919 and 1921 popularized and elaborated Lewis's model. Langmuir subsequently introduced the term ''covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between ato ...
''. In 1921, Otto Stern
:''Otto Stern was also the pen name of German women's rights activist Louise Otto-Peters (1819–1895)''.
Otto Stern (; 17 February 1888 – 17 August 1969) was a German-American physicist and Nobel laureate in physics. He was the second most ...
and Walther Gerlach established the concept of quantum mechanical spin in subatomic particles.
For cases where no sharing was involved, Lewis in 1923 developed the electron pair theory of acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a se ...
s and base: Lewis redefined an acid as any atom or molecule with an incomplete octet that was thus capable of accepting electrons from another atom; bases were, of course, electron donors. His theory is known as the concept of Lewis acids and bases. In 1923, G. N. Lewis and Merle Randall
Merle Randall (January 29, 1888 – March 17, 1950) was an American physical chemist famous for his work with Gilbert N. Lewis, over a period of 25 years, in measuring reaction heat of chemical compounds and determining their corresponding free ...
published ''Thermodynamics and the Free Energy of Chemical Substances'', first modern treatise on chemical thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws ...
.
The 1920s saw a rapid adoption and application of Lewis's model of the electron-pair bond in the fields of organic and coordination chemistry. In organic chemistry, this was primarily due to the efforts of the British chemists Arthur Lapworth, Robert Robinson, Thomas Lowry, and Christopher Ingold; while in coordination chemistry, Lewis's bonding model was promoted through the efforts of the American chemist Maurice Huggins and the British chemist Nevil Sidgwick.
Quantum mechanics
In 1924, French quantum physicist Louis de Broglie published his thesis, in which he introduced a revolutionary theory of electron waves based on wave–particle duality
Wave–particle duality is the concept in quantum mechanics that every particle or quantum entity may be described as either a particle or a wave. It expresses the inability of the classical physics, classical concepts "particle" or "wave" to fu ...
. In his time, the wave and particle interpretations of light and matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic part ...
were seen as being at odds with one another, but de Broglie suggested that these seemingly different characteristics were instead the same behavior observed from different perspectives — that particles can behave like waves, and waves (radiation) can behave like particles. Broglie's proposal offered an explanation of the restricted motion of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
s within the atom. The first publications of Broglie's idea of "matter waves" had drawn little attention from other physicists, but a copy of his doctoral thesis chanced to reach Einstein, whose response was enthusiastic. Einstein stressed the importance of Broglie's work both explicitly and by building further on it.
In 1925, Austrian-born physicist Wolfgang Pauli
Wolfgang Ernst Pauli (; ; 25 April 1900 – 15 December 1958) was an Austrian theoretical physicist and one of the pioneers of quantum physics. In 1945, after having been nominated by Albert Einstein, Pauli received the Nobel Prize in Physics ...
developed the Pauli exclusion principle
In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins (i.e. fermions) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formula ...
, which states that no two electrons around a single nucleus in an atom can occupy the same quantum state
In quantum physics, a quantum state is a mathematical entity that provides a probability distribution for the outcomes of each possible measurement on a system. Knowledge of the quantum state together with the rules for the system's evolution i ...
simultaneously, as described by four quantum numbers. Pauli made major contributions to quantum mechanics and quantum field theory – he was awarded the 1945 Nobel Prize for Physics for his discovery of the Pauli exclusion principle – as well as solid-state physics, and he successfully hypothesized the existence of the neutrino. In addition to his original work, he wrote masterful syntheses of several areas of physical theory that are considered classics of scientific literature.
In 1926 at the age of 39, Austrian theoretical physicist Erwin Schrödinger produced the papers that gave the foundations of quantum wave mechanics. In those papers he described his partial differential equation that is the basic equation of quantum mechanics and bears the same relation to the mechanics of the atom as Newton's equations of motion bear to planetary astronomy. Adopting a proposal made by Louis de Broglie in 1924 that particles of matter have a dual nature and in some situations act like waves, Schrödinger introduced a theory describing the behaviour of such a system by a wave equation that is now known as the Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
. The solutions to Schrödinger's equation, unlike the solutions to Newton's equations, are wave functions that can only be related to the probable occurrence of physical events. The readily visualized sequence of events of the planetary orbits of Newton is, in quantum mechanics, replaced by the more abstract notion of probability
Probability is the branch of mathematics concerning numerical descriptions of how likely an event is to occur, or how likely it is that a proposition is true. The probability of an event is a number between 0 and 1, where, roughly speaking, ...
. (This aspect of the quantum theory made Schrödinger and several other physicists profoundly unhappy, and he devoted much of his later life to formulating philosophical objections to the generally accepted interpretation of the theory that he had done so much to create.)
German theoretical physicist Werner Heisenberg
Werner Karl Heisenberg () (5 December 1901 – 1 February 1976) was a German theoretical physicist and one of the main pioneers of the theory of quantum mechanics. He published his work in 1925 in a Über quantentheoretische Umdeutung kinematis ...
was one of the key creators of quantum mechanics. In 1925, Heisenberg discovered a way to formulate quantum mechanics in terms of matrices. For that discovery, he was awarded the Nobel Prize for Physics for 1932. In 1927 he published his uncertainty principle, upon which he built his philosophy and for which he is best known. Heisenberg was able to demonstrate that if you were studying an electron in an atom you could say where it was (the electron's location) or where it was going (the electron's velocity), but it was impossible to express both at the same time. He also made important contributions to the theories of the hydrodynamics
In physics and engineering, fluid dynamics is a subdiscipline of fluid mechanics that describes the flow of fluids— liquids and gases. It has several subdisciplines, including ''aerodynamics'' (the study of air and other gases in motion) a ...
of turbulent flows, the atomic nucleus, ferromagnetism, cosmic rays
Cosmic rays are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our ...
, and subatomic particle
In physical sciences, a subatomic particle is a particle that composes an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles (for example, a p ...
s, and he was instrumental in planning the first West German nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
at Karlsruhe
Karlsruhe ( , , ; South Franconian: ''Kallsruh'') is the third-largest city of the German state (''Land'') of Baden-Württemberg after its capital of Stuttgart and Mannheim, and the 22nd-largest city in the nation, with 308,436 inhabitants. ...
, together with a research reactor in Munich, in 1957. Considerable controversy surrounds his work on atomic research during World War II.
Quantum chemistry
Some view the birth of quantum chemistry in the discovery of the Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
and its application to the hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen cons ...
in 1926. However, the 1927 article of Walter Heitler
Walter Heinrich Heitler (; 2 January 1904 – 15 November 1981) was a German physicist who made contributions to quantum electrodynamics and quantum field theory. He brought chemistry under quantum mechanics through his theory of valence bo ...
and Fritz London is often recognised as the first milestone in the history of quantum chemistry. This is the first application of quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, ...
to the diatomic hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
molecule, and thus to the phenomenon of the chemical bond. In the following years much progress was accomplished by Edward Teller, Robert S. Mulliken, Max Born
Max Born (; 11 December 1882 – 5 January 1970) was a German physicist and mathematician who was instrumental in the development of quantum mechanics. He also made contributions to solid-state physics and optics and supervised the work of a ...
, J. Robert Oppenheimer, Linus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific topi ...
, Erich Hückel
Erich Armand Arthur Joseph Hückel (August 9, 1896, Berlin – February 16, 1980, Marburg) was a German physicist and physical chemist. He is known for two major contributions:
*The Debye–Hückel theory of electrolytic solutions
*The Hücke ...
, Douglas Hartree
Douglas Rayner Hartree (27 March 1897 – 12 February 1958) was an English mathematician and physicist most famous for the development of numerical analysis and its application to the Hartree–Fock equations of atomic physics and the c ...
and Vladimir Aleksandrovich Fock, to cite a few.
Still, skepticism remained as to the general power of quantum mechanics applied to complex chemical systems. The situation around 1930 is described by Paul Dirac
Paul Adrien Maurice Dirac (; 8 August 1902 – 20 October 1984) was an English theoretical physicist who is regarded as one of the most significant physicists of the 20th century. He was the Lucasian Professor of Mathematics at the Univer ...
:
Hence the quantum mechanical methods developed in the 1930s and 1940s are often referred to as theoretical molecular
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
or atomic physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. Atomic physics typically refers to the study of atomic structure and the interaction between atoms. It is primarily concerned wit ...
to underline the fact that they were more the application of quantum mechanics to chemistry and spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter ...
than answers to chemically relevant questions. In 1951, a milestone article in quantum chemistry is the seminal paper of Clemens C. J. Roothaan on Roothaan equations The Roothaan equations are a representation of the Hartree–Fock equation in a non orthonormal basis set which can be of Gaussian-type or Slater-type. It applies to closed-shell molecules or atoms where all molecular orbitals or atomic orbita ...
. It opened the avenue to the solution of the self-consistent field
In classical deductive logic, a consistent theory is one that does not lead to a logical contradiction. The lack of contradiction can be defined in either semantic or syntactic terms. The semantic definition states that a theory is consistent i ...
equations for small molecules like hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
or nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
. Those computations were performed with the help of tables of integrals which were computed on the most advanced computers of the time.
In the 1940s many physicists turned from molecular
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
or atomic physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. Atomic physics typically refers to the study of atomic structure and the interaction between atoms. It is primarily concerned wit ...
to nuclear physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies t ...
(like J. Robert Oppenheimer or Edward Teller). Glenn T. Seaborg was an American nuclear chemist best known for his work on isolating and identifying transuranium elements (those heavier than uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
). He shared the 1951 Nobel Prize for Chemistry with Edwin Mattison McMillan for their independent discoveries of transuranium elements. Seaborgium was named in his honour, making him the only person, along with Albert Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theor ...
and Yuri Oganessian, for whom a chemical element was named during his lifetime.
Molecular biology and biochemistry
By the mid 20th century, in principle, the integration of physics and chemistry was extensive, with chemical properties explained as the result of the electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
ic structure of the atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, a ...
; Linus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific topi ...
's book on ''The Nature of the Chemical Bond'' used the principles of quantum mechanics to deduce bond angles in ever-more complicated molecules. However, though some principles deduced from quantum mechanics were able to predict qualitatively some chemical features for biologically relevant molecules, they were, till the end of the 20th century, more a collection of rules, observations, and recipes than rigorous ab initio quantitative methods.
 This heuristic approach triumphed in 1953 when
This heuristic approach triumphed in 1953 when James Watson
James Dewey Watson (born April 6, 1928) is an American molecular biologist, geneticist, and zoologist. In 1953, he co-authored with Francis Crick the academic paper proposing the double helix structure of the DNA molecule. Watson, Crick a ...
and Francis Crick
Francis Harry Compton Crick (8 June 1916 – 28 July 2004) was an English molecular biologist, biophysicist, and neuroscientist. He, James Watson, Rosalind Franklin, and Maurice Wilkins played crucial roles in deciphering the helical stru ...
deduced the double helical structure of DNA by constructing models constrained by and informed by the knowledge of the chemistry of the constituent parts and the X-ray diffraction patterns obtained by Rosalind Franklin
Rosalind Elsie Franklin (25 July 192016 April 1958) was a British chemist and X-ray crystallographer whose work was central to the understanding of the molecular structures of DNA (deoxyribonucleic acid), RNA (ribonucleic acid), viruses, ...
. This discovery lead to an explosion of research into the biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
of life.
In the same year, the Miller–Urey experiment demonstrated that basic constituents of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
, simple amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
s, could themselves be built up from simpler molecules in a simulation
A simulation is the imitation of the operation of a real-world process or system over time. Simulations require the use of Conceptual model, models; the model represents the key characteristics or behaviors of the selected system or proc ...
of primordial processes
A process is a series or set of activities that interact to produce a result; it may occur once-only or be recurrent or periodic.
Things called a process include:
Business and management
*Business process, activities that produce a specific se ...
on Earth. This first attempt by chemists to study hypothetical processes in the laboratory under controlled conditions helped kickstart bountiful research, within the natural sciences
Natural science is one of the branches of science concerned with the description, understanding and prediction of natural phenomena, based on empirical evidence from observation and experimentation. Mechanisms such as peer review and repeat ...
, into the origins of life
In biology, abiogenesis (from a- 'not' + Greek bios 'life' + genesis 'origin') or the origin of life is the natural process by which life has arisen from non-living matter, such as simple organic compounds. The prevailing scientific hypot ...
.
In 1983 Kary Mullis devised a method for the in-vitro amplification of DNA, known as the polymerase chain reaction
The polymerase chain reaction (PCR) is a method widely used to rapidly make millions to billions of copies (complete or partial) of a specific DNA sample, allowing scientists to take a very small sample of DNA and amplify it (or a part of it) ...
(PCR), which revolutionized the chemical processes used in the laboratory to manipulate it. PCR could be used to synthesize specific pieces of DNA and made possible the sequencing of DNA of organisms, which culminated in the huge human genome project
The Human Genome Project (HGP) was an international scientific research project with the goal of determining the base pairs that make up human DNA, and of identifying, mapping and sequencing all of the genes of the human genome from both ...
.
An important piece in the double helix puzzle was solved by one of Pauling's students Matthew Meselson
Matthew Stanley Meselson (born May 24, 1930) is a geneticist and molecular biologist currently at Harvard University, known for his demonstration, with Franklin Stahl, of semi-conservative DNA replication. After completing his Ph.D. under Li ...
and Frank Stahl, the result of their collaboration ( Meselson–Stahl experiment) has been called as "the most beautiful experiment in biology".
They used a centrifugation technique that sorted molecules according to differences in weight. Because nitrogen atoms are a component of DNA, they were labelled and therefore tracked in replication in bacteria.
Late 20th century
 In 1970, John Pople developed the
In 1970, John Pople developed the Gaussian
Carl Friedrich Gauss (1777–1855) is the eponym of all of the topics listed below.
There are over 100 topics all named after this German mathematician and scientist, all in the fields of mathematics, physics, and astronomy. The English eponym ...
program greatly easing computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into computer programs, to calculate the structures and properties of mo ...
calculations. In 1971, Yves Chauvin offered an explanation of the reaction mechanism of olefin metathesis reactions. In 1975, Karl Barry Sharpless and his group discovered stereoselective oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
reactions including Sharpless epoxidation
The Sharpless epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols. The oxidizing agent is ''tert''-butyl hydroperoxide. The method relies on a catalyst formed fro ...
, Sharpless asymmetric dihydroxylation, and Sharpless oxyamination.
In 1985, Harold Kroto, Robert Curl and Richard Smalley discovered fullerenes, a class of large carbon molecules superficially resembling the geodesic dome designed by architect R. Buckminster Fuller. In 1991, Sumio Iijima used electron microscopy
An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination. As the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, electron microscopes have a hi ...
to discover a type of cylindrical fullerene known as a carbon nanotube
A scanning tunneling microscopy image of a single-walled carbon nanotube
Rotating single-walled zigzag carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers.
''Single-wall carbon na ...
, though earlier work had been done in the field as early as 1951. This material is an important component in the field of nanotechnology
Nanotechnology, also shortened to nanotech, is the use of matter on an atomic, molecular, and supramolecular scale for industrial purposes. The earliest, widespread description of nanotechnology referred to the particular technological goal ...
. In 1994, K. C. Nicolaou
Kyriacos Costa Nicolaou ( el, Κυριάκος Κ. Νικολάου; born July 5, 1946) is a Cypriot-American chemist known for his research in the area of natural products total synthesis. He is currently Harry C. and Olga K. Wiess Professor of ...
with his group and Robert A. Holton
Robert A. Holton (born 1944) is an American academic chemist who is known for his work regarding the chemical synthesis for Taxol (known as the Holton Taxol total synthesis), a widely utilized and highly effective anti-cancer drug. He is a Profe ...
and his group, achieved the first total synthesis of Taxol. In 1995, Eric Cornell and Carl Wieman
Carl Edwin Wieman (born March 26, 1951) is an American physicist and educationist at Stanford University, and currently the A.D White Professor at Large at Cornell University. In 1995, while at the University of Colorado Boulder, he and Eric All ...
produced the first Bose–Einstein condensate, a substance that displays quantum mechanical properties on the macroscopic scale.
Mathematics and chemistry
Before the 20th century, chemistry was defined as the science of the nature of matter and its transformations. It was therefore distinct from physics which was not concerned with such dramatic transformation of matter. Moreover, in contrast to physics, chemistry remained predominantly a descriptive and empirical science until the end of the 19th century. Though they developed a consistent quantitative foundation based on notions of atomic and molecular weights, combining proportions, and thermodynamic quantities, chemists had less use of advanced mathematics.Auguste Comte
Isidore Marie Auguste François Xavier Comte (; 19 January 1798 – 5 September 1857) was a French philosopher and writer who formulated the doctrine of positivism. He is often regarded as the first philosopher of science in the modern sense ...
wrote in 1830:
However, in the second part of the 19th century, the situation began to change as August Kekulé wrote in 1867:
Scope of chemistry
As understanding of the nature of matter has evolved, so too has the self-understanding of the science of chemistry by its practitioners. This continuing historical process of evaluation includes the categories, terms, aims and scope of chemistry. Additionally, the development of the social institutions and networks which support chemical enquiry are highly significant factors that enable the production, dissemination and application of chemical knowledge. (See Philosophy of chemistry)
Chemical industry
The later part of the nineteenth century saw a huge increase in the exploitation of petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
extracted from the earth for the production of a host of chemicals and largely replaced the use of whale oil
Whale oil is oil obtained from the blubber of whales. Whale oil from the bowhead whale was sometimes known as train oil, which comes from the Dutch word ''traan'' (" tear" or "drop").
Sperm oil, a special kind of oil obtained from the head ...
, coal tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat pso ...
and naval stores used previously. Large-scale production and refinement of petroleum provided feedstocks for liquid fuels such as gasoline
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organic c ...
and diesel
Diesel may refer to:
* Diesel engine, an internal combustion engine where ignition is caused by compression
* Diesel fuel, a liquid fuel used in diesel engines
* Diesel locomotive, a railway locomotive in which the prime mover is a diesel engi ...
, solvents, lubricants
A lubricant (sometimes shortened to lube) is a substance that helps to reduce friction between surfaces in mutual contact, which ultimately reduces the heat generated when the surfaces move. It may also have the function of transmitting forces, t ...
, asphalt
Asphalt, also known as bitumen (, ), is a sticky, black, highly viscous liquid or semi-solid form of petroleum. It may be found in natural deposits or may be a refined product, and is classed as a pitch. Before the 20th century, the term ...
, waxes
Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low ...
, and for the production of many of the common materials of the modern world, such as synthetic fibers
Fiber or fibre (from la, fibra, links=no) is a natural or artificial substance that is significantly longer than it is wide. Fibers are often used in the manufacture of other materials. The strongest engineering materials often incorporate ...
, plastics, paints, detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are m ...
s, pharmaceuticals
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the medical field and re ...
, adhesives and ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
as fertilizer
A fertilizer (American English) or fertiliser (British English; see spelling differences) is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from ...
and for other uses. Many of these required new catalysts and the utilization of chemical engineering
Chemical engineering is an engineering field which deals with the study of operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials in ...
for their cost-effective production.
In the mid-twentieth century, control of the electronic structure of semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way ...
materials was made precise by the creation of large ingots of extremely pure single crystals of silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
and germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors ...
. Accurate control of their chemical composition by doping with other elements made the production of the solid state transistor
upright=1.4, gate (G), body (B), source (S) and drain (D) terminals. The gate is separated from the body by an insulating layer (pink).
A transistor is a semiconductor device used to Electronic amplifier, amplify or electronic switch, switch ...
in 1951 and made possible the production of tiny integrated circuit
An integrated circuit or monolithic integrated circuit (also referred to as an IC, a chip, or a microchip) is a set of electronic circuits on one small flat piece (or "chip") of semiconductor material, usually silicon. Large numbers of tiny ...
s for use in electronic devices, especially computers.
See also
Histories and timelines
* Atomic theory
Atomic theory is the scientific theory that matter is composed of particles called atoms. Atomic theory traces its origins to an ancient philosophical tradition known as atomism. According to this idea, if one were to take a lump of matter ...
* Cupellation
* History of chromatography
* History of electrochemistry
* History of the molecule
* History of molecular biology The history of molecular biology begins in the 1930s with the convergence of various, previously distinct biological and physical disciplines: biochemistry, genetics, microbiology, virology and physics. With the hope of understanding life at it ...
* History of physics
* History of science and technology
The history of science and technology (HST) is a field of history that examines the understanding of the natural world (science) and the ability to manipulate it ( technology) at different points in time. This academic discipline also studies the ...
* History of the periodic table
The periodic table is an arrangement of the chemical elements, structured by their atomic number, electron configuration and recurring chemical properties. In the basic form, elements are presented in order of increasing atomic number, in the r ...
* History of thermodynamics
The history of thermodynamics is a fundamental strand in the history of physics, the history of chemistry, and the history of science in general. Owing to the relevance of thermodynamics in much of science and technology, its history is finel ...
* History of energy
* History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.
The modern concept of molecules can be traced back towards pre-scientific and Greek ph ...
* History of materials science
* List of years in science
__NOTOC__
The following entries cover events related to science or technology which occurred in the listed year.
Before 2000s
* 0s: 1st century in science
* 100s: 2nd century in science
* 200s: 3rd century in science
* 300s: 4th century in s ...
* Nobel Prize in chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
* Timeline of atomic and subatomic physics
A timeline of atomic and subatomic physics.
Early beginnings
*In 6th century BCE, Acharya Kanada proposed that all matter must consist of indivisible particles and called them "anu". He proposes examples like ripening of fruit as the change in ...
* Timeline of chemical elements discoveries
* Timeline of chemistry
This timeline of chemistry lists important works, discoveries, ideas, inventions, and experiments that significantly changed humanity's understanding of the modern science known as chemistry, defined as the scientific study of the composition of ...
* Timeline of materials technology
* Timeline of thermodynamics, statistical mechanics, and random processes
* The Chemical History of a Candle
* The Mystery of Matter: Search for the Elements (PBS film)
Notable chemists
''listed chronologically:''
* List of chemists
* Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders ...
, 1627–1691
* Joseph Black, 1728–1799
* Joseph Priestley
Joseph Priestley (; 24 March 1733 – 6 February 1804) was an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted ...
, 1733–1804
* Carl Wilhelm Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hydr ...
, 1742–1786
* Antoine Lavoisier, 1743–1794
* Alessandro Volta
Alessandro Giuseppe Antonio Anastasio Volta (, ; 18 February 1745 – 5 March 1827) was an Italian physicist, chemist and lay Catholic who was a pioneer of electricity and power who is credited as the inventor of the electric battery and th ...
, 1745–1827
* Jacques Charles, 1746–1823
* Claude Louis Berthollet, 1748–1822
* Amedeo Avogadro, 1776–1856
* Joseph-Louis Gay-Lussac, 1778–1850
* Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for ...
, 1778–1829
* Jöns Jacob Berzelius
Baron Jöns Jacob Berzelius (; by himself and his contemporaries named only Jacob Berzelius, 20 August 1779 – 7 August 1848) was a Swedish chemist. Berzelius is considered, along with Robert Boyle, John Dalton, and Antoine Lavoisier, to be o ...
, inventor of modern chemical notation, 1779–1848
* Justus von Liebig
Justus Freiherr von Liebig (12 May 1803 – 20 April 1873) was a German scientist who made major contributions to agricultural and biological chemistry, and is considered one of the principal founders of organic chemistry. As a professor at th ...
, 1803–1873
* Louis Pasteur
Louis Pasteur (, ; 27 December 1822 – 28 September 1895) was a French chemist and microbiologist renowned for his discoveries of the principles of vaccination, microbial fermentation and pasteurization, the latter of which was named afte ...
, 1822–1895
* Stanislao Cannizzaro, 1826–1910
* Friedrich August Kekulé von Stradonitz Friedrich may refer to:
Names
* Friedrich (surname), people with the surname ''Friedrich''
* Friedrich (given name), people with the given name ''Friedrich''
Other
* Friedrich (board game), a board game about Frederick the Great and the Seven Year ...
, 1829–1896
* Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
, 1834–1907
* Josiah Willard Gibbs
Josiah Willard Gibbs (; February 11, 1839 – April 28, 1903) was an American scientist who made significant theoretical contributions to physics, chemistry, and mathematics. His work on the applications of thermodynamics was instrumental in t ...
, 1839–1903
* J. H. van 't Hoff, 1852–1911
* William Ramsay, 1852–1916
* Svante Arrhenius, 1859–1927
* Walther Nernst, 1864–1941
* Marie Curie, 1867–1934
* Gilbert N. Lewis
Gilbert Newton Lewis (October 23 or October 25, 1875 – March 23, 1946) was an American physical chemist and a Dean of the College of Chemistry at University of California, Berkeley. Lewis was best known for his discovery of the covalent bond a ...
, 1875–1946
* Otto Hahn, 1879–1968
* Irving Langmuir
Irving Langmuir (; January 31, 1881 – August 16, 1957) was an American chemist, physicist, and engineer. He was awarded the Nobel Prize in Chemistry in 1932 for his work in surface chemistry.
Langmuir's most famous publication is the 1919 ar ...
, 1881–1957
* Linus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific topi ...
, 1901–1994
* Glenn T. Seaborg, 1912–1999
* Robert Burns Woodward, 1917–1979
* Frederick Sanger, 1918–2013
* Geoffrey Wilkinson, 1921–1996
* Rudolph A. Marcus, 1923–
* George Andrew Olah, 1926–2017
* Elias James Corey, 1928–
* Akira Suzuki (chemist), Akira Suzuki, 1930–
* Richard F. Heck, 1931–2015
* Harold Kroto, 1939–2016
* Jean-Marie Lehn, 1939–
* Peter Atkins, 1940–
* Barry Sharpless, 1941–
* Richard Smalley, 1943–2005
* Jean-Pierre Sauvage, 1944–
Notes
References
Selected classic papers from the history of chemistry
*Eric R. Scerri, The Periodic Table: Its Story and Its Significance, Oxford University Press, 2006.
Further reading
*
*
* John Servos, Servos, John W.
''Physical chemistry from Ostwald to Pauling : the making of a science in America''
Princeton, N.J. : Princeton University Press, 1990.
; Documentaries
* BBC (2010). ''Chemistry: A Volatile History''.
External links
ChemisLab
– Chemists of the Past
SHAC: Society for the History of Alchemy and Chemistry
{{DEFAULTSORT:History Of Chemistry
History of chemistry,
History of science, Chemistry
History of science by discipline, Chemistry
History of industries, Chemistry
 The history of chemistry represents a time span from
The history of chemistry represents a time span from  Philosophical attempts to rationalize why different substances have different properties (color, density, smell), exist in different states (gaseous, liquid, and solid), and react in a different manner when exposed to environments, for example to water or fire or temperature changes, led ancient philosophers to postulate the first theories on nature and chemistry. The history of such philosophical theories that relate to chemistry can probably be traced back to every single ancient civilization. The common aspect in all these theories was the attempt to identify a small number of primary classical elements that make up all the various substances in nature. Substances like air, water, and soil/earth, energy forms, such as fire and light, and more abstract concepts such as thoughts, aether, and heaven, were common in ancient civilizations even in the absence of any cross-fertilization: for example ancient Greek, Indian, Mayan, and Chinese philosophies all considered air,
Philosophical attempts to rationalize why different substances have different properties (color, density, smell), exist in different states (gaseous, liquid, and solid), and react in a different manner when exposed to environments, for example to water or fire or temperature changes, led ancient philosophers to postulate the first theories on nature and chemistry. The history of such philosophical theories that relate to chemistry can probably be traced back to every single ancient civilization. The common aspect in all these theories was the attempt to identify a small number of primary classical elements that make up all the various substances in nature. Substances like air, water, and soil/earth, energy forms, such as fire and light, and more abstract concepts such as thoughts, aether, and heaven, were common in ancient civilizations even in the absence of any cross-fertilization: for example ancient Greek, Indian, Mayan, and Chinese philosophies all considered air,  The elemental system used in medieval
The elemental system used in medieval  Alchemy is defined by the Hermetic quest for the philosopher's stone, the study of which is steeped in symbolic mysticism, and differs greatly from modern science. Alchemists toiled to make transformations on an
Alchemy is defined by the Hermetic quest for the philosopher's stone, the study of which is steeped in symbolic mysticism, and differs greatly from modern science. Alchemists toiled to make transformations on an  Practical attempts to improve the refining of ores and their extraction to smelt metals was an important source of information for early chemists in the 16th century, among them
Practical attempts to improve the refining of ores and their extraction to smelt metals was an important source of information for early chemists in the 16th century, among them 
 Anglo-Irish chemist
Anglo-Irish chemist  In 1702, German chemist Georg Stahl coined the name " phlogiston" for the substance believed to be released in the process of burning. Around 1735, Swedish chemist Georg Brandt analyzed a dark blue pigment found in copper ore. Brandt demonstrated that the pigment contained a new element, later named
In 1702, German chemist Georg Stahl coined the name " phlogiston" for the substance believed to be released in the process of burning. Around 1735, Swedish chemist Georg Brandt analyzed a dark blue pigment found in copper ore. Brandt demonstrated that the pigment contained a new element, later named  Antoine-Laurent de Lavoisier demonstrated with careful measurements that transmutation of water to earth was not possible, but that the sediment observed from boiling water came from the container. He burnt phosphorus and sulfur in air, and proved that the products weighed more than the original samples, with the mass gained being lost from the air. Thus, in 1789, he established the Law of Conservation of Mass, which is also called "Lavoisier's Law."
Repeating the experiments of Priestley, he demonstrated that air is composed of two parts, one of which combines with metals to form calxes. In ''Considérations Générales sur la Nature des Acides'' (1778), he demonstrated that the "air" responsible for combustion was also the source of acidity. The next year, he named this portion oxygen (Greek for acid-former), and the other azote (Greek for no life). Because of his more thorough characterization of it as an element, Lavoisier thus has a claim to the discovery of oxygen along with Priestley and Scheele. He also discovered that the "inflammable air" discovered by Cavendish – which he termed
Antoine-Laurent de Lavoisier demonstrated with careful measurements that transmutation of water to earth was not possible, but that the sediment observed from boiling water came from the container. He burnt phosphorus and sulfur in air, and proved that the products weighed more than the original samples, with the mass gained being lost from the air. Thus, in 1789, he established the Law of Conservation of Mass, which is also called "Lavoisier's Law."
Repeating the experiments of Priestley, he demonstrated that air is composed of two parts, one of which combines with metals to form calxes. In ''Considérations Générales sur la Nature des Acides'' (1778), he demonstrated that the "air" responsible for combustion was also the source of acidity. The next year, he named this portion oxygen (Greek for acid-former), and the other azote (Greek for no life). Because of his more thorough characterization of it as an element, Lavoisier thus has a claim to the discovery of oxygen along with Priestley and Scheele. He also discovered that the "inflammable air" discovered by Cavendish – which he termed 
 In 1803, English meteorologist and chemist
In 1803, English meteorologist and chemist  A Swedish chemist and disciple of Dalton,
A Swedish chemist and disciple of Dalton,  English chemist
English chemist  French chemist Joseph Louis Gay-Lussac shared the interest of Lavoisier and others in the quantitative study of the properties of gases. From his first major program of research in 1801–1802, he concluded that equal volumes of all gases expand equally with the same increase in temperature: this conclusion is usually called " Charles's law", as Gay-Lussac gave credit to Jacques Charles, who had arrived at nearly the same conclusion in the 1780s but had not published it.
French chemist Joseph Louis Gay-Lussac shared the interest of Lavoisier and others in the quantitative study of the properties of gases. From his first major program of research in 1801–1802, he concluded that equal volumes of all gases expand equally with the same increase in temperature: this conclusion is usually called " Charles's law", as Gay-Lussac gave credit to Jacques Charles, who had arrived at nearly the same conclusion in the 1780s but had not published it. After Dalton published his atomic theory in 1808, certain of his central ideas were soon adopted by most chemists. However, uncertainty persisted for half a century about how atomic theory was to be configured and applied to concrete situations; chemists in different countries developed several different incompatible atomistic systems. A paper that suggested a way out of this difficult situation was published as early as 1811 by the Italian physicist Amedeo Avogadro (1776–1856), who hypothesized that equal volumes of gases at the same
After Dalton published his atomic theory in 1808, certain of his central ideas were soon adopted by most chemists. However, uncertainty persisted for half a century about how atomic theory was to be configured and applied to concrete situations; chemists in different countries developed several different incompatible atomistic systems. A paper that suggested a way out of this difficult situation was published as early as 1811 by the Italian physicist Amedeo Avogadro (1776–1856), who hypothesized that equal volumes of gases at the same  Avogadro's hypothesis began to gain broad appeal among chemists only after his compatriot and fellow scientist Stanislao Cannizzaro demonstrated its value in 1858, two years after Avogadro's death. Cannizzaro's chemical interests had originally centered on natural products and on reactions of
Avogadro's hypothesis began to gain broad appeal among chemists only after his compatriot and fellow scientist Stanislao Cannizzaro demonstrated its value in 1858, two years after Avogadro's death. Cannizzaro's chemical interests had originally centered on natural products and on reactions of  British chemist and physicist William Crookes is noted for his cathode ray studies, fundamental in the development of
British chemist and physicist William Crookes is noted for his cathode ray studies, fundamental in the development of 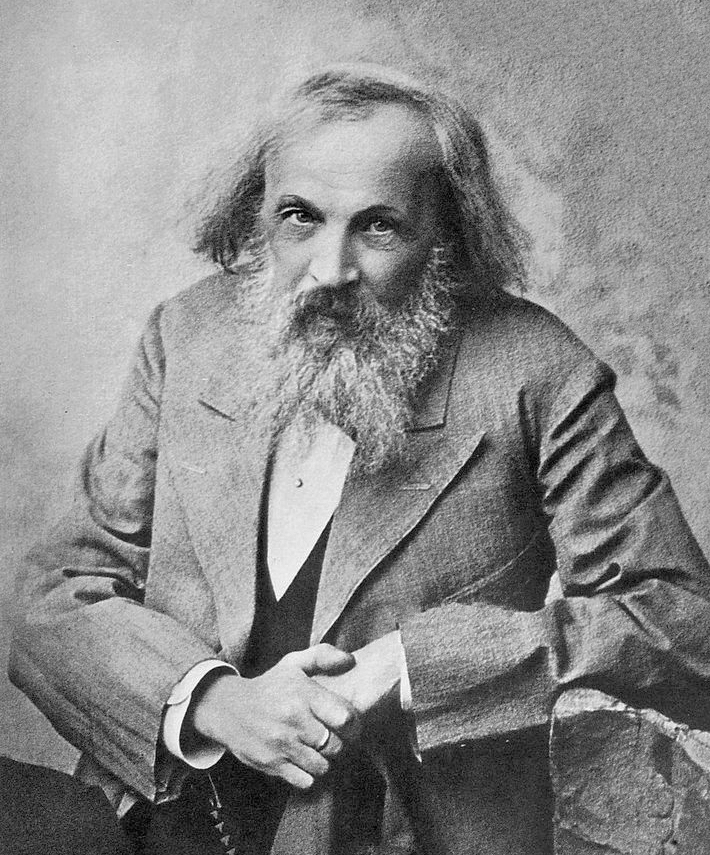 An important breakthrough in making sense of the list of known chemical elements (as well as in understanding the internal structure of atoms) was
An important breakthrough in making sense of the list of known chemical elements (as well as in understanding the internal structure of atoms) was  American mathematical physicist J. Willard Gibbs's work on the applications of
American mathematical physicist J. Willard Gibbs's work on the applications of  The following year, Ramsay liberated another inert gas from a mineral called
The following year, Ramsay liberated another inert gas from a mineral called 
 Pierre and Marie further explored radioactivity by working to separate the substances in uranium ores and then using the electrometer to make radiation measurements to ‘trace’ the minute amount of unknown radioactive element among the fractions that resulted. Working with the mineral pitchblende, the pair discovered a new radioactive element in 1898. They named the element polonium, after Marie's native country of Poland. On December 21, 1898, the Curies detected the presence of another radioactive material in the pitchblende. They presented this finding to the
Pierre and Marie further explored radioactivity by working to separate the substances in uranium ores and then using the electrometer to make radiation measurements to ‘trace’ the minute amount of unknown radioactive element among the fractions that resulted. Working with the mineral pitchblende, the pair discovered a new radioactive element in 1898. They named the element polonium, after Marie's native country of Poland. On December 21, 1898, the Curies detected the presence of another radioactive material in the pitchblende. They presented this finding to the  New Zealand-born chemist and physicist Ernest Rutherford is considered to be "the father of
New Zealand-born chemist and physicist Ernest Rutherford is considered to be "the father of  In 1903, Mikhail Tsvet invented chromatography, an important analytic technique. In 1904, Hantaro Nagaoka proposed an early nuclear model of the atom, where electrons orbit a dense massive nucleus. In 1905, Fritz Haber and Carl Bosch developed the Haber process for making
In 1903, Mikhail Tsvet invented chromatography, an important analytic technique. In 1904, Hantaro Nagaoka proposed an early nuclear model of the atom, where electrons orbit a dense massive nucleus. In 1905, Fritz Haber and Carl Bosch developed the Haber process for making  In 1905,
In 1905,  In 1913, Niels Bohr, a Danish physicist, introduced the concepts of
In 1913, Niels Bohr, a Danish physicist, introduced the concepts of  In 1913, Henry Moseley, working from Van den Broek's earlier idea, introduced the concept of atomic number to fix some inadequacies of Mendeleev's periodic table, which had been based on atomic weight. The peak of Frederick Soddy's career in radiochemistry was in 1913 with his formulation of the concept of
In 1913, Henry Moseley, working from Van den Broek's earlier idea, introduced the concept of atomic number to fix some inadequacies of Mendeleev's periodic table, which had been based on atomic weight. The peak of Frederick Soddy's career in radiochemistry was in 1913 with his formulation of the concept of