half-sandwich compound on:
[Wikipedia]
[Google]
[Amazon]
Half sandwich compounds, also known as piano stool complexes, are
MMT-2D-skeletal.png, MMT is a commercially useful antiknock compound.
Cpco(CO)2.png, CpCo(CO)2 is a catalyst for the synthesis of pyridines.
Cyclobutadienyl-iron-tricarbonyl-from-xtal-3D-balls.png, (C4H4)Fe(CO)3.
Cp2Fe(CO)2I-2D-skeletal.png, CpFe(CO)2I is an example of a piano stool complex with two different monodentate ligands.
RuCymCl2.png, The diruthenium of cymene is readily cleaved by ligands to give monoRu half-sandwich derivatives.
CHTMo(CO)3.png, Cycloheptatriene molybdenum tricarbonyl
CPPCDV01.png, Cp2V2(CO)5 featuring a pair of semi-bridging CO ligands.

 (''η''6-C6H6)Cr(CO)3 is a useful
(''η''6-C6H6)Cr(CO)3 is a useful

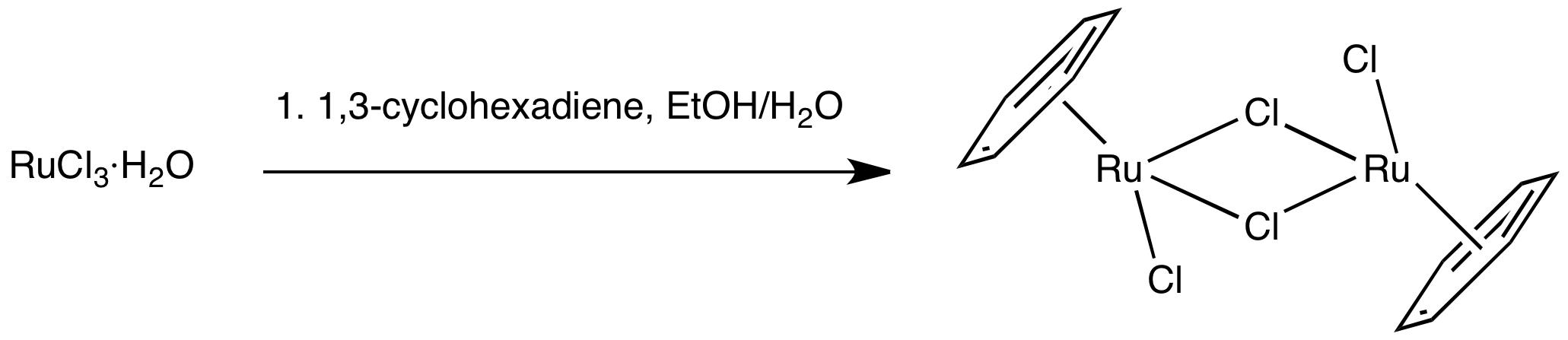 (''η''6-C6H6)RuCl2 readily undergoes ligand exchange via cleavage of the chloride bridges, making this complex a versatile precursor to Ru(II) piano stool derivatives.
(''η''6-C6H6)RuCl2 readily undergoes ligand exchange via cleavage of the chloride bridges, making this complex a versatile precursor to Ru(II) piano stool derivatives.
organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
complexes that feature a cyclic polyhapto ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl
Cyclobutadieneiron tricarbonyl is an organoiron compound with the formula Fe(C4H4)(CO)3. It is a yellow solid that is soluble in organic solvents. It has been used in organic chemistry as a precursor for cyclobutadiene, which is an elusive specie ...
and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid w ...
s, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent
An antiknock agent is a gasoline additive used to reduce engine knocking and increase the fuel's octane rating by raising the temperature and pressure at which auto-ignition occurs. The mixture known as gasoline or petrol, when used in high com ...
in petrol
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organic c ...
.
(''η''5-C5H5) piano stool compounds
Half sandwich complexes containing cyclopentadienyl ligands are common. Well studied examples include (''η''5-C5H5)V(CO)4, (''η''5-C5H5)Cr(CO)3H, (''η''5-CH3C5H4)Mn(CO)3, (''η''5-C5H5)Cr(CO)3H, ''η''5-C5H5)Fe(CO)3sup>+, (''η''5-C5H5)V(CO)4I, and (''η''5-C5H5)Ru(NCMe). (''η''5-C5H5)Co(CO)2 is a two-legged piano stool complex. Bulky cyclopentadienyl ligands such as 1,2,4-C5H2(''tert''-Bu)3− form unusual half-sandwich complexes.(''η''6-C6H6) piano stool compounds
Inorganometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and s ...
, (''η''6-C6H6) piano stool compounds are half-sandwich compounds with (''η''6-C6H6)ML3 structure (M = Cr, Mo, W, Mn(I), Re(I) and L = typically CO). (''η''6-C6H6) piano stool complexes are stable 18-electron coordination compounds with a variety of chemical and material applications. Early studies on (''η''6-C6H6)Cr(CO)3 were carried out by Natta, Ercoli and Calderazzo, and Fischer and Ofele, and the crystal structure was determined by Corradini and Allegra in 1959. The X-ray data indicate that the plane of the benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
ring is nearly parallel to the plane defined by the oxygen atoms of the carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
ligands, and so the structure resembles a benzene seat mounted on three carbonyl legs tethered by the metal atom.
Cr and Mn(I) (''η''6-C6H6) piano stool complexes
Piano stool complexes of the type (''η''6-C6H6)M(CO)3 are typically synthesized by heating the appropriatemetal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe c ...
compound with benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
. Alternately, the same compounds can be obtained by carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbon ...
of the bis(arene) sandwich compounds, such as (''η''6-C6H6)2M compound with the metal carbonyl compound. This second approach may be more appropriate for arene
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past groupin ...
ligands containing thermally fragile substituents.
:
Reactivity of (''η''6-C6H6)Cr(CO)3
The benzene ligand in (''η''6-C6H6)Cr(CO)3Mi is prone to deprotonation. For example,Organolithium compound
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s form adducts featuring cyclohexadienyl ligands. Subsequent oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
of the complex results in the release of a substituted benzene. Oxidation of the chromium atom by I2 and other iodine reagents has been shown to promote exchange of arene ligands, but the intermediate chromium iodide species has not been characterized.
:
(''η''6-C6H6)Cr(CO)3 complexes exhibit "''cine''" and "''tele''" nucleophilic aromatic addition. Processes of this type involve reaction of (''η''6-C6H6)Cr(CO)3 with an alkyl lithium reagent. Subsequent treatment with an acid results in the addition of a nucleophile to the benzene ring at a site ''ortho'' ("''cine''"), ''meta'' or ''para'' ("''tele''") to the ''ipso'' carbon (see Arene substitution patterns
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.
''Ortho'', ''meta'', and ''para'' substitution
* I ...
).
:
Reflecting its increased acidity, the benzene ligand can be lithiated with ''n''-butyllithium. The resulting organolithium compound
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
serves as a nucleophile in various reactions, for example, with trimethylsilyl chloride
Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. I ...
:
: (''η''6-C6H6)Cr(CO)3 is a useful
(''η''6-C6H6)Cr(CO)3 is a useful catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
for the hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic ...
of 1,3-diene
In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. ...
s. The product alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic ...
results from 1,4-addition of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
. The complex does not hydrogenate isolated double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
s.
A variety of arenes ligands have been installed aside from benzene. Weakly coordinating ligands
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
may be employed to improve ligand exchange and thus the turnover rates for (''η''6-C6H6)M(CO)3 complexes.(''η''6-C6H6)M(CO)3 complexes have been incorporated into high surface area porous
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure ...
materials.
(''η''6-C6H6)M(CO)3 complexes serve as models for the interaction of metal carbonyls with graphene
Graphene () is an allotrope of carbon consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice nanostructure.
and carbon nanotube
A scanning tunneling microscopy image of a single-walled carbon nanotube
Rotating single-walled zigzag carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers.
''Single-wall carbon na ...
s. The presence of M(CO)3 on extended π-network materials has been shown to improve electrical conductivity across the material.
Reactivity of ''η''6-C6H6)Mn(CO)3sup>+
Typical arene tricarbonyl piano stool complexes of Mn(I) and Re(I) are cationic and thus exhibit enhanced reactivity toward nucleophiles. Subsequent to nucleophilic addition, the modified arene can be recovered from the metal. :
(''η''6-C6H6)Ru complexes
Half-sandwich compounds employing Ru(II), such as (cymene)ruthenium dichloride dimer, have been mainly investigated as catalysts for transfer hydrogenation. These complexes feature three coordination sites that are susceptible to substitution, while the arene ligand is tightly bonded and protects the metal against oxidation to Ru(III). They are prepared by reaction of RuCl3·''x''(H2O) with1,3-cyclohexadiene
Cyclohexa-1,3-diene is an organic compound with the formula (C2H4)(CH)4. It is a colorless, flammable liquid. Its refractive index is 1.475 (20 °C, D). A naturally occurring derivative of 1,3-cyclohexadiene is terpinene, a component of pine o ...
s. Work is also conducted on their potential as anticancer drugs.
: (''η''6-C6H6)RuCl2 readily undergoes ligand exchange via cleavage of the chloride bridges, making this complex a versatile precursor to Ru(II) piano stool derivatives.
(''η''6-C6H6)RuCl2 readily undergoes ligand exchange via cleavage of the chloride bridges, making this complex a versatile precursor to Ru(II) piano stool derivatives.
References
{{organometallics Organic compounds Organometallic chemistry Coordination chemistry