glycogen phosphorylase on:
[Wikipedia]
[Google]
[Amazon]
Glycogen phosphorylase is one of the phosphorylase
 Glycogen phosphorylase can act only on
Glycogen phosphorylase can act only on 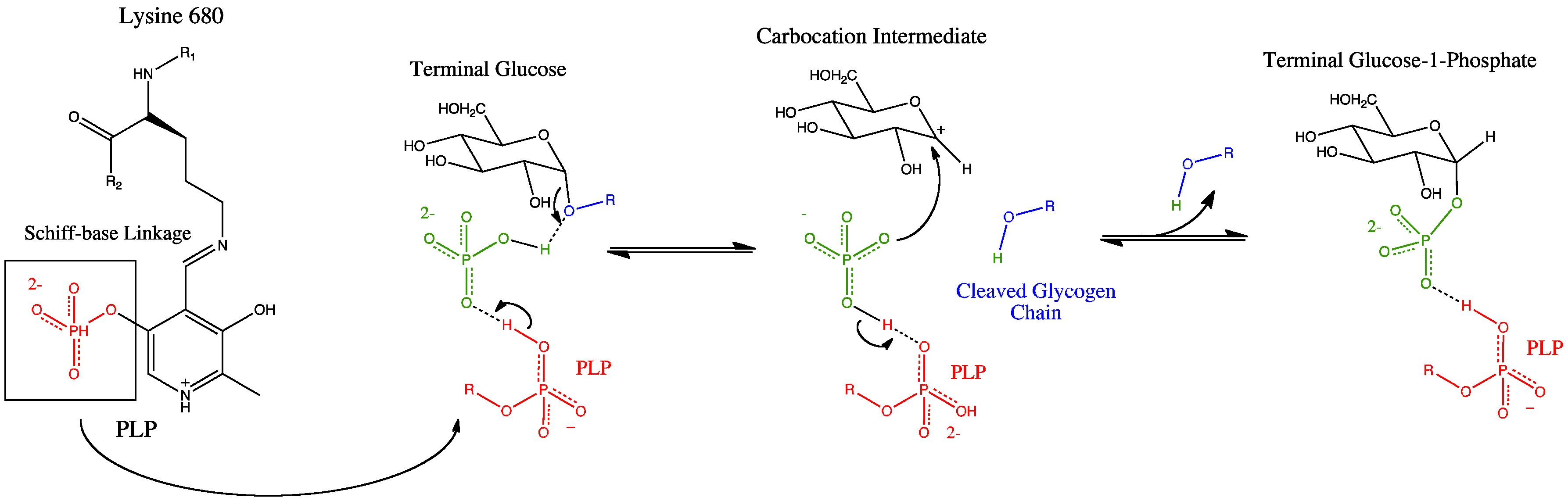
GeneReviews/NCBI/NIH/UW entry on Glycogen Storage Disease Type VI - Hers disease
* * * * {{DEFAULTSORT:Glycogen Phosphorylase EC 2.4.1
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s (). Glycogen phosphorylase catalyzes the rate-limiting step in glycogenolysis
Glycogenolysis is the breakdown of glycogen (n) to glucose-1-phosphate and glycogen (n-1). Glycogen branches are catabolized by the sequential removal of glucose monomers via phosphorolysis, by the enzyme glycogen phosphorylase.
Mechanism
T ...
in animals by releasing glucose-1-phosphate from the terminal alpha-1,4-glycosidic bond. Glycogen phosphorylase is also studied as a model protein regulated by both reversible phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
and allosteric
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site.
The site to which the effector binds is termed the ''allosteric sit ...
effects.
Mechanism
Glycogen phosphorylase breaks upglycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body.
Glycogen functions as one of ...
into glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
subunits (see also figure below):
(α-1,4 glycogen chain)n + Pi ⇌ (α-1,4 glycogen chain)n-1 + α-D-glucose-1-phosphate.
Glycogen is left with one fewer glucose molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and b ...
, and the free glucose molecule is in the form of glucose-1-phosphate. In order to be used for metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run ...
, it must be converted to glucose-6-phosphate by the enzyme phosphoglucomutase.
Although the reaction is reversible in vitro
''In vitro'' (meaning in glass, or ''in the glass'') studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called " test-tube experiments", these studies in biology a ...
, within the cell the enzyme only works in the forward direction as shown below because the concentration of inorganic phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
is much higher than that of glucose-1-phosphate.
 Glycogen phosphorylase can act only on
Glycogen phosphorylase can act only on linear
Linearity is the property of a mathematical relationship ('' function'') that can be graphically represented as a straight line. Linearity is closely related to '' proportionality''. Examples in physics include rectilinear motion, the linear ...
chains of glycogen (α1-4 glycosidic linkage). Its work will immediately come to a halt four residues away from α1-6 branch
A branch, sometimes called a ramus in botany, is a woody structural member connected to the central trunk of a tree (or sometimes a shrub). Large branches are known as boughs and small branches are known as twigs. The term '' twig'' usuall ...
(which are exceedingly common in glycogen). In these situations, the debranching enzyme is necessary, which will straighten out the chain in that area. In addition, the enzyme transferase shifts a block of 3 glucosyl residues from the outer branch to the other end, and then a α1-6 glucosidase enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
is required to break the remaining (single glucose) α1-6 residue that remains in the new linear chain. After all this is done, glycogen phosphorylase can continue. The enzyme is specific to α1-4 chains, as the molecule contains a 30-angstrom-long crevice with the same radius as the helix formed by the glycogen chain; this accommodates 4-5 glucosyl residues, but is too narrow for branches. This crevice connects the glycogen storage site to the active, catalytic site.
Glycogen phosphorylase has a pyridoxal phosphate
Pyridoxal phosphate (PLP, pyridoxal 5'- phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent ...
(PLP, derived from Vitamin B6) at each catalytic site. Pyridoxal phosphate links with basic residues (in this case Lys680) and covalently forms a Schiff base. Once the Schiff base linkage is formed, holding the PLP molecule in the active site, the phosphate group on the PLP readily donates a proton to an inorganic phosphate molecule, allowing the inorganic phosphate to in turn be deprotonated by the oxygen forming the α-1,4 glycosidic linkage. PLP is readily deprotonated because its negative charge is not only stabilized within the phosphate group, but also in the pyridine ring, thus the conjugate base resulting from the deprotonation of PLP is quite stable. The protonated oxygen now represents a good leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited ...
, and the glycogen chain is separated from the terminal glycogen in an SN1 fashion, resulting in the formation of a glucose molecule with a secondary carbocation at the 1 position. Finally, the deprotonated inorganic phosphate acts as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
and bonds with the carbocation, resulting in the formation of glucose-1-phosphate and a glycogen chain shortened by one glucose molecule.
There is also an alternative proposed mechanism involving a positively charged oxygen in a half-chair conformation.

Structure
The glycogen phosphorylase monomer is a large protein, composed of 842 amino acids with a mass of 97.434kDa
The dalton or unified atomic mass unit (symbols: Da or u) is a non-SI unit of mass widely used in physics and chemistry. It is defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at re ...
in muscle cells. While the enzyme can exist as an inactive monomer or tetramer, it is biologically active as a dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ( ...
of two identical subunits.
In mammals, the major isozyme In biochemistry, isozymes (also known as isoenzymes or more generally as multiple forms of enzymes) are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. Isozymes usually have different kinetic parameters (e.g. dif ...
s of glycogen phosphorylase are found in muscle, liver, and brain. The brain type is predominant in adult brain and embryonic tissues, whereas the liver and muscle types are predominant in adult liver and skeletal muscle, respectively.
The glycogen phosphorylase dimer has many regions of biological significance, including catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
sites, glycogen binding sites, allosteric
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site.
The site to which the effector binds is termed the ''allosteric sit ...
sites, and a reversibly phosphorylated serine residue. First, the catalytic sites are relatively buried, 15Å from the surface of the protein and from the subunit interface. This lack of easy access of the catalytic site to the surface is significant in that it makes the protein activity highly susceptible to regulation, as small allosteric effects could greatly increase the relative access of glycogen to the site.
Perhaps the most important regulatory site
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site.
The site to which the effector binds is termed the ''allosteric site ...
is Ser14, the site of reversible phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
very close to the subunit interface. The structural change associated with phosphorylation, and with the conversion of phosphorylase b to phosphorylase a, is the arrangement of the originally disordered residues 10 to 22 into α helices. This change increases phosphorylase activity up to 25% even in the absence of AMP, and enhances AMP activation further.
The allosteric site of AMP binding on muscle isoforms of glycogen phosphorylase are close to the subunit interface just like Ser14. Binding of AMP at this site, corresponding in a change from the T state of the enzyme to the R state, results in small changes in tertiary structure at the subunit interface leading to large changes in quaternary structure. AMP binding rotates the tower helices (residues 262-278) of the two subunits 50˚ relative to one another through greater organization and intersubunit interactions. This rotation of the tower helices leads to a rotation of the two subunits by 10˚ relative to one another, and more importantly disorders residues 282-286 (the 280s loop) that block access to the catalytic site in the T state but do not in the R state.
The final, perhaps most curious site on the glycogen phosphorylase protein is the so-called glycogen storage site. Residues 397-437 form this structure, which allows the protein to covalently bind to the glycogen chain a full 30 Å from the catalytic site . This site is most likely the site at which the enzyme binds to glycogen granules before initiating cleavage of terminal glucose molecules. In fact, 70% of dimeric phosphorylase in the cell exists as bound to glycogen granules rather than free floating.
Clinical significance
The inhibition of glycogen phosphorylase has been proposed as one method for treatingtype 2 diabetes
Type 2 diabetes, formerly known as adult-onset diabetes, is a form of diabetes mellitus that is characterized by high blood sugar, insulin resistance, and relative lack of insulin. Common symptoms include increased thirst, frequent urinatio ...
. Since glucose production in the liver has been shown to increase in type 2 diabetes patients, inhibiting the release of glucose from the liver's glycogen's supplies appears to be a valid approach. The cloning of the human liver glycogen phosphorylase (HLGP) revealed a new allosteric binding site near the subunit interface that is not present in the rabbit muscle glycogen phosphorylase (RMGP) normally used in studies. This site was not sensitive to the same inhibitors as those at the AMP allosteric site, and most success has been had synthesizing new inhibitors that mimic the structure of glucose, since glucose-6-phosphate is a known inhibitor of HLGP and stabilizes the less active T-state. These glucose derivatives have had some success in inhibiting HLGP, with predicted Ki values as low as 0.016 mM.
Mutations in the muscle isoform of glycogen phosphorylase (PYGM) are associated with glycogen storage disease type V
Glycogen storage disease type V (GSD5, GSD-V), also known as McArdle's disease, is a metabolic disorder, one of the metabolic myopathies, more specifically a muscle glycogen storage disease, caused by a deficiency of myophosphorylase. Its inci ...
(GSD V, McArdle's Disease). More than 65 mutations in the PYGM gene that lead to McArdle disease have been identified to date. Symptoms of McArdle disease include muscle weakness, myalgia
Myalgia (also called muscle pain and muscle ache in layman's terms) is the medical term for muscle pain. Myalgia is a symptom of many diseases. The most common cause of acute myalgia is the overuse of a muscle or group of muscles; another li ...
, and lack of endurance, all stemming from low glucose levels in muscle tissue.
Mutations in the liver isoform of glycogen phosphorylase (PYGL) are associated with Hers' Disease (glycogen storage disease type VI
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. The polysaccharide structure represents the main storage form of glucose in the body.
Glycogen functions as one of ...
). Hers' disease is often associated with mild symptoms normally limited to hypoglycemia
Hypoglycemia, also called low blood sugar, is a fall in blood sugar to levels below normal, typically below 70 mg/dL (3.9 mmol/L). Whipple's triad is used to properly identify hypoglycemic episodes. It is defined as blood glucose bel ...
, and is sometimes difficult to diagnose due to residual enzyme activity.
The brain isoform of glycogen phosphorylase (PYGB) has been proposed as a biomarker
In biomedical contexts, a biomarker, or biological marker, is a measurable indicator of some biological state or condition. Biomarkers are often measured and evaluated using blood, urine, or soft tissues to examine normal biological processes, p ...
for gastric cancer
Stomach cancer, also known as gastric cancer, is a cancer that develops from the lining of the stomach. Most cases of stomach cancers are gastric carcinomas, which can be divided into a number of subtypes, including gastric adenocarcinomas. Lym ...
.
Regulation
Glycogen phosphorylase is regulated throughallosteric
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site.
The site to which the effector binds is termed the ''allosteric sit ...
control and through phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, wh ...
. Phosphorylase a and phosphorylase b each exist in two forms a T (tense) inactive state and R (relaxed) state. Phosphorylase b is normally in the T state, inactive due to the physiological presence of ATP and Glucose 6 phosphate, and Phosphorylase a is normally in the R state (active). An isoenzyme of glycogen phosphorylase exists in the liver sensitive to glucose concentration, as the liver acts as a glucose exporter. In essence, liver phosphorylase is responsive to glucose, which causes a very responsive transition from the R to T form, inactivating it; furthermore, liver phosphorylase is insensitive to AMP.
Hormones such as epinephrine
Adrenaline, also known as epinephrine, is a hormone and medication which is involved in regulating visceral functions (e.g., respiration). It appears as a white microcrystalline granule. Adrenaline is normally produced by the adrenal glands and ...
, insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the ''INS'' gene. It is considered to be the main anabolic hormone of the body. It regulates the metabolism ...
and glucagon
Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It raises concentration of glucose and fatty acids in the bloodstream, and is considered to be the main catabolic hormone of the body. It is also used as a medication to tre ...
regulate glycogen phosphorylase using second messenger amplification systems linked to G proteins
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their ac ...
. Glucagon activates adenylate cyclase through a G protein-coupled receptor (GPCR) coupled to Gs which in turn activates adenylate cyclase to increase intracellular concentrations of cAMP. cAMP binds to and activates protein kinase A
In cell biology, protein kinase A (PKA) is a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase (). PKA has several functions in the cell, including regulatio ...
(PKA). PKA phosphorylates phosphorylase kinase
Phosphorylase kinase (PhK) is a serine/threonine-specific protein kinase which activates glycogen phosphorylase to release glucose-1-phosphate from glycogen. PhK phosphorylates glycogen phosphorylase at two serine residues, triggering a conformatio ...
, which in turn phosphorylates glycogen phosphorylase b at Ser14, converting it into the active glycogen phosphorylase a.
In the liver, glucagon
Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It raises concentration of glucose and fatty acids in the bloodstream, and is considered to be the main catabolic hormone of the body. It is also used as a medication to tre ...
also activates another GPCR that triggers a different cascade, resulting in the activation of phospholipase C (PLC). PLC indirectly causes the release of calcium from the hepatocytes' endoplasmic reticulum into the cytosol. The increased calcium availability binds to the calmodulin
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the bin ...
subunit and activates glycogen phosphorylase kinase. Glycogen phosphorylase kinase activates glycogen phosphorylase in the same manner mentioned previously.
Glycogen phosphorylase b is not always inactive in muscle, as it can be activated allosterically by AMP. An increase in AMP concentration, which occurs during strenuous exercise, signals energy demand. AMP activates glycogen phosphorylase b by changing its conformation from a tense to a relaxed form. This relaxed form has similar enzymatic properties as the phosphorylated enzyme. An increase in ATP concentration opposes this activation by displacing AMP from the nucleotide binding site, indicating sufficient energy stores.
Upon eating a meal, there is a release of insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the ''INS'' gene. It is considered to be the main anabolic hormone of the body. It regulates the metabolism ...
, signaling glucose availability in the blood. Insulin indirectly activates protein phosphatase 1 (PP1) and phosphodiesterase
A phosphodiesterase (PDE) is an enzyme that breaks a phosphodiester bond. Usually, ''phosphodiesterase'' refers to cyclic nucleotide phosphodiesterases, which have great clinical significance and are described below. However, there are many ot ...
via a signal transduction cascade. PP1 dephosphorylates glycogen phosphorylase a, reforming the inactive glycogen phosphorylase b. The phosphodiesterase converts cAMP to AMP. Together, they decrease the concentration of cAMP and inhibit PKA. As a result, PKA can no longer initiate the phosphorylation cascade that ends with formation of (active) glycogen phosphorylase a. Overall, insulin signaling decreases glycogenolysis to preserve glycogen stores in the cell and triggers glycogenesis
Glycogenesis is the process of glycogen synthesis, in which glucose molecules are added to chains of glycogen for storage. This process is activated during rest periods following the Cori cycle, in the liver, and also activated by insulin in ...
.
Historical significance
Glycogen phosphorylase was the first allosteric enzyme to be discovered. It was isolated and its activity characterized in detail by Carl F. Cori, Gerhard Schmidt and Gerty T. Cory.Arda Green
Arda Alden Green (May 7, 1899 – January 22, 1958) was an American biochemist who co-discovered the neurotransmitter serotonin and discovered the reaction responsible for firefly bioluminescence. She is also known for contributing to Gerty Cor ...
and Gerty Cori
Gerty Theresa Cori (; August 15, 1896 – October 26, 1957) was an Austro-Hungarian and American biochemist who in 1947 was the third woman to win a Nobel Prize in science, and the first woman to be awarded the Nobel Prize in Physiology or Me ...
crystallized it for the first time in 1943 and illustrated that glycogen phosphorylase existed in either the or b forms depending on its phosphorylation state, as well as in the R or T states based on the presence of AMP.
See also
* AMP deaminase deficiency (MADD) *Glycogenolysis
Glycogenolysis is the breakdown of glycogen (n) to glucose-1-phosphate and glycogen (n-1). Glycogen branches are catabolized by the sequential removal of glucose monomers via phosphorolysis, by the enzyme glycogen phosphorylase.
Mechanism
T ...
* McArdle Disease (GSD-V)
* Metabolic myopathies
* Purine Nucleotide Cycle § Pathology
References
Further reading
* * * *External links
GeneReviews/NCBI/NIH/UW entry on Glycogen Storage Disease Type VI - Hers disease
* * * * {{DEFAULTSORT:Glycogen Phosphorylase EC 2.4.1