Fermium on:
[Wikipedia]
[Google]
[Amazon]
Fermium is a


 Fermium was first discovered in the fallout from the '
Fermium was first discovered in the fallout from the '
. Retrieved 2 December 2007 Initial examination of the debris from the explosion had shown the production of a new isotope of
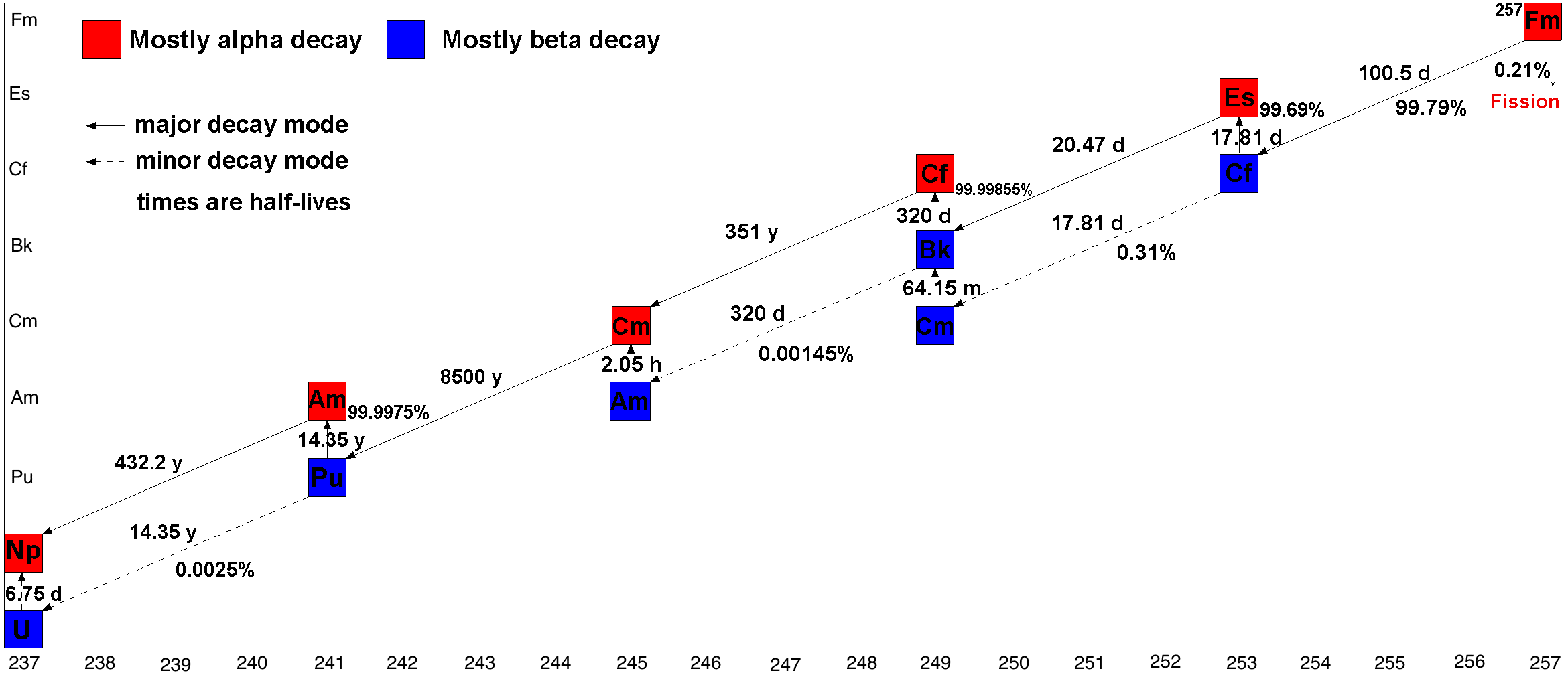 There are 20 isotopes of fermium listed in NUBASE 2016, with atomic weights of 241 to 260, of which 257Fm is the longest-lived with a
There are 20 isotopes of fermium listed in NUBASE 2016, with atomic weights of 241 to 260, of which 257Fm is the longest-lived with a
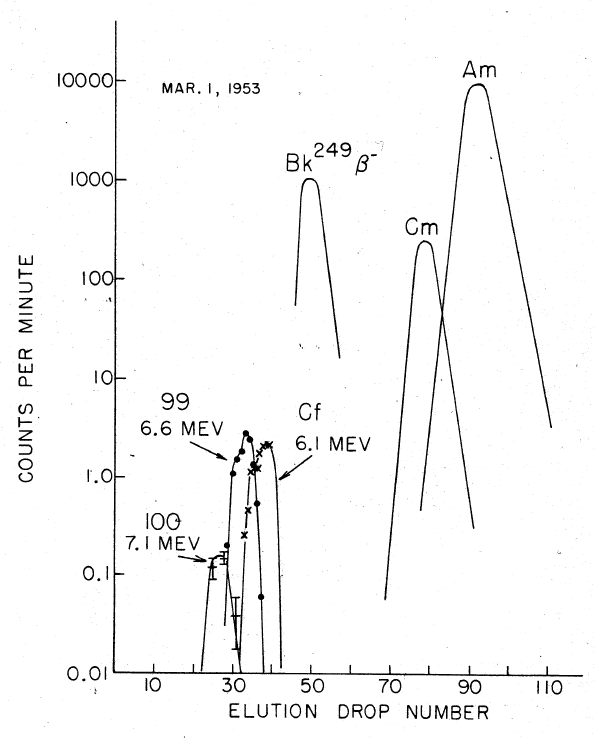 Fermium is produced by the bombardment of lighter
Fermium is produced by the bombardment of lighter
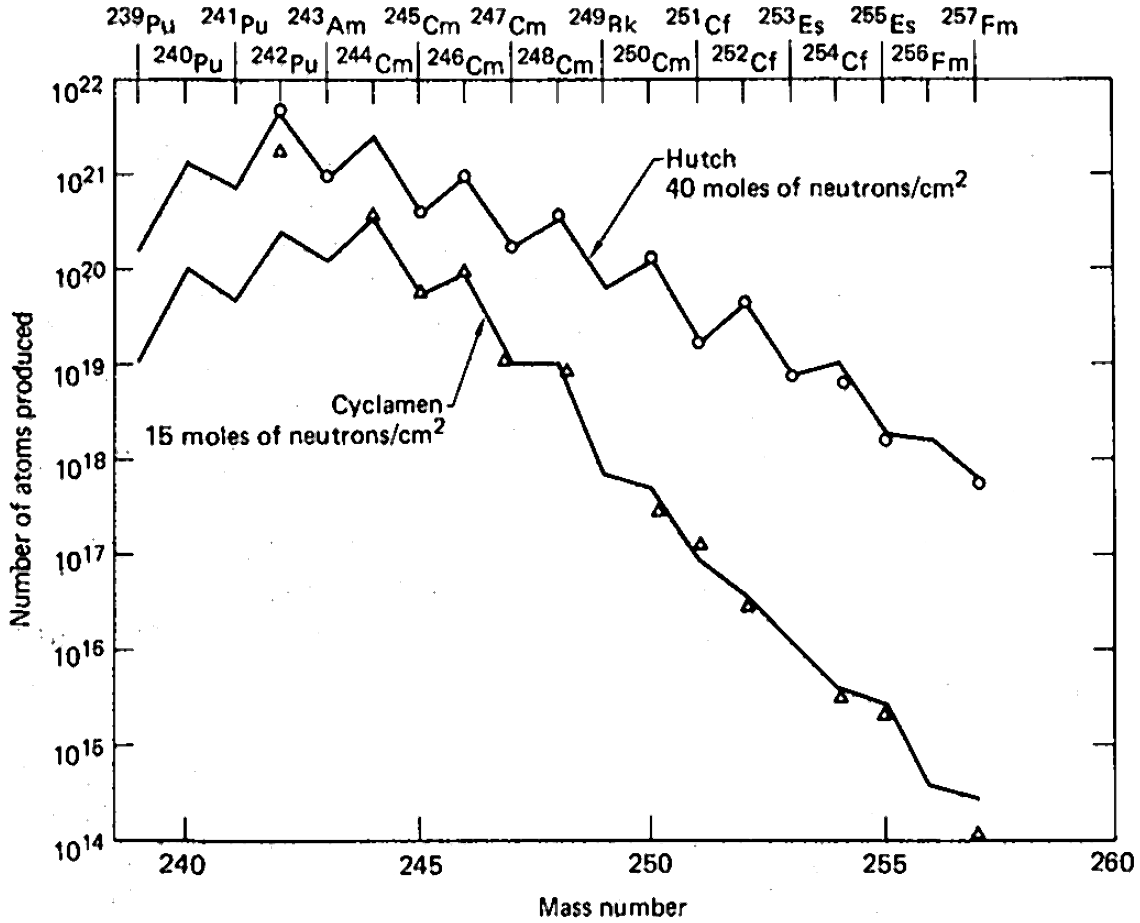 The atmospheric results were supplemented by the underground test data accumulated in the 1960s at the
The atmospheric results were supplemented by the underground test data accumulated in the 1960s at the
 The chemistry of fermium has only been studied in solution using tracer techniques, and no solid compounds have been prepared. Under normal conditions, fermium exists in solution as the Fm3+ ion, which has a
The chemistry of fermium has only been studied in solution using tracer techniques, and no solid compounds have been prepared. Under normal conditions, fermium exists in solution as the Fm3+ ion, which has a
Fermium, Mendelevium, Nobelium, and Lawrencium
in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Hrsg.): ''The Chemistry of the Actinide and Transactinide Elements'', Springer, Dordrecht 2006; , p. 1621–1651; . * Seaborg, Glenn T. (ed.) (1978)
Proceedings of the Symposium Commemorating the 25th Anniversary of Elements 99 and 100
', 23 January 1978, Report LBL-7701 * '' Gmelins Handbuch der anorganischen Chemie'', System Nr. 71, Transurane: Teil A 1 II, p. 19–20; Teil A 2, p. 47; Teil B 1, p. 84.
Fermium
at ''
synthetic element
A synthetic element is one of 24 known chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Fm and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
100. It is an actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
and the heaviest element that can be formed by neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
bombardment of lighter elements, and hence the last element that can be prepared in macroscopic quantities, although pure fermium metal has not yet been prepared. A total of 19 isotopes are known, with 257Fm being the longest-lived with a half-life of 100.5 days.
It was discovered in the debris of the first hydrogen bomb
A thermonuclear weapon, fusion weapon or hydrogen bomb (H bomb) is a second-generation nuclear weapon design. Its greater sophistication affords it vastly greater destructive power than first-generation nuclear bombs, a more compact size, a lowe ...
explosion in 1952, and named after Enrico Fermi
Enrico Fermi (; 29 September 1901 – 28 November 1954) was an Italian (later naturalized American) physicist and the creator of the world's first nuclear reactor, the Chicago Pile-1. He has been called the "architect of the nuclear age" an ...
, one of the pioneers of nuclear physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies t ...
. Its chemistry is typical for the late actinides, with a preponderance of the +3 oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
but also an accessible +2 oxidation state. Owing to the small amounts of produced fermium and all of its isotopes having relatively short half-lives, there are currently no uses for it outside basic scientific research.
Discovery


 Fermium was first discovered in the fallout from the '
Fermium was first discovered in the fallout from the 'Ivy Mike
Ivy Mike was the codename given to the first full-scale test of a thermonuclear device, in which part of the explosive yield comes from nuclear fusion.
Ivy Mike was detonated on November 1, 1952, by the United States on the island of Elugelab ...
' nuclear test (1 November 1952), the first successful test of a hydrogen bomb.Fermium – National Research Council Canada. Retrieved 2 December 2007 Initial examination of the debris from the explosion had shown the production of a new isotope of
plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
, : this could only have formed by the absorption of six neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
s by a uranium-238
Uranium-238 (238U or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However ...
nucleus followed by two β− decays. At the time, the absorption of neutrons by a heavy nucleus was thought to be a rare process, but the identification of raised the possibility that still more neutrons could have been absorbed by the uranium nuclei, leading to new elements.
Element 99 (einsteinium
Einsteinium is a synthetic element with the symbol Es and atomic number 99. Einsteinium is a member of the actinide series and it is the seventh transuranium element. It was named in honor of Albert Einstein.
Einsteinium was discovered as a com ...
) was quickly discovered on filter papers which had been flown through the cloud from the explosion (the same sampling technique that had been used to discover ). It was then identified in December 1952 by Albert Ghiorso
Albert Ghiorso (July 15, 1915 – December 26, 2010) was an American nuclear scientist and co-discoverer of a record 12 chemical elements on the periodic table. His research career spanned six decades, from the early 1940s to the late 1990s.
Biog ...
and co-workers at the University of California at Berkeley
The University of California, Berkeley (UC Berkeley, Berkeley, Cal, or California) is a public university, public land-grant university, land-grant research university in Berkeley, California. Established in 1868 as the University of Californi ...
. They discovered the isotope 253Es (half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
20.5 days) that was made by the capture
Capture may refer to:
*Asteroid capture, a phenomenon in which an asteroid enters a stable orbit around another body
*Capture, a software for lighting design, documentation and visualisation
*"Capture" a song by Simon Townshend
*Capture (band), an ...
of 15 neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
s by uranium-238
Uranium-238 (238U or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However ...
nuclei – which then underwent seven successive beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
s:
Some 238U atoms, however, could capture another amount of neutrons (most likely, 16 or 17). The discovery of fermium (''Z'' = 100) required more material, as the yield was expected to be at least an order of magnitude lower than that of element 99, and so contaminated coral from the
Enewetak atoll
Enewetak Atoll (; also spelled Eniwetok Atoll or sometimes Eniewetok; mh, Ānewetak, , or , ; known to the Japanese as Brown Atoll or Brown Island; ja, ブラウン環礁) is a large coral atoll of 40 islands in the Pacific Ocean and with it ...
(where the test had taken place) was shipped to the University of California Radiation Laboratory
Lawrence Berkeley National Laboratory (LBNL), commonly referred to as the Berkeley Lab, is a United States national laboratory that is owned by, and conducts scientific research on behalf of, the United States Department of Energy. Located in ...
in Berkeley, California
Berkeley ( ) is a city on the eastern shore of San Francisco Bay in northern Alameda County, California, United States. It is named after the 18th-century Irish bishop and philosopher George Berkeley. It borders the cities of Oakland and E ...
, for processing and analysis. About two months after the test, a new component was isolated emitting high-energy α-particles (7.1 MeV) with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of about a day. With such a short half-life, it could only arise from the β− decay of an isotope of einsteinium, and so had to be an isotope of the new element 100: it was quickly identified as 255Fm ().
The discovery of the new elements, and the new data on neutron capture, was initially kept secret on the orders of the U.S. military until 1955 due to Cold War
The Cold War is a term commonly used to refer to a period of geopolitical tension between the United States and the Soviet Union and their respective allies, the Western Bloc and the Eastern Bloc. The term '' cold war'' is used because t ...
tensions. Nevertheless, the Berkeley team was able to prepare elements 99 and 100 by civilian means, through the neutron bombardment of plutonium-239
Plutonium-239 (239Pu or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three mai ...
, and published this work in 1954 with the disclaimer that it was not the first studies that had been carried out on the elements. The "Ivy Mike" studies were declassified and published in 1955.
The Berkeley team had been worried that another group might discover lighter isotopes of element 100 through ion-bombardment techniques before they could publish their classified research, and this proved to be the case. A group at the Nobel Institute for Physics in Stockholm independently discovered the element, producing an isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
later confirmed to be 250Fm (''t''1/2 = 30 minutes) by bombarding a target with oxygen-16 ions, and published their work in May 1954. Nevertheless, the priority of the Berkeley team was generally recognized, and with it the prerogative to name the new element in honour of Enrico Fermi
Enrico Fermi (; 29 September 1901 – 28 November 1954) was an Italian (later naturalized American) physicist and the creator of the world's first nuclear reactor, the Chicago Pile-1. He has been called the "architect of the nuclear age" an ...
, the developer of the first artificial self-sustained nuclear reactor. Fermi was still alive when the name was proposed, but had died by the time it became official.
Isotopes
half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of 100.5 days. 253Fm has a half-life of 3 days, while 251Fm of 5.3 h, 252Fm of 25.4 h, 254Fm of 3.2 h, 255Fm of 20.1 h, and 256Fm of 2.6 hours. All the remaining ones have half-lives ranging from 30 minutes to less than a millisecond.
The neutron capture product of fermium-257, 258Fm, undergoes spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakd ...
with a half-life of just 370(14) microseconds; 259Fm and 260Fm are also unstable with respect to spontaneous fission (''t''1/2 = 1.5(3) s and 4 ms respectively). This means that neutron capture cannot be used to create nuclide
A nuclide (or nucleide, from atomic nucleus, nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was co ...
s with a mass number
The mass number (symbol ''A'', from the German word ''Atomgewicht'' tomic weight, also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approxima ...
greater than 257, unless carried out in a nuclear explosion. As 257Fm is an α-emitter, decaying to 253Cf, and no known fermium isotopes undergo beta minus decay to the next element, mendelevium
Mendelevium is a synthetic element with the symbol Md ( formerly Mv) and atomic number 101. A metallic radioactive transuranium element in the actinide series, it is the first element by atomic number that currently cannot be produced in macrosco ...
, fermium is also the last element that can be prepared by a neutron-capture process. Because of this impediment in forming heavier isotopes, these short-lived isotopes 258–260Fm constitute the so-called "fermium gap."
Production
 Fermium is produced by the bombardment of lighter
Fermium is produced by the bombardment of lighter actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
s with neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
s in a nuclear reactor. Fermium-257 is the heaviest isotope that is obtained via neutron capture, and can only be produced in picogram quantities. The major source is the 85 MW High Flux Isotope Reactor
The High Flux Isotope Reactor (HFIR) is a nuclear research reactor at Oak Ridge National Laboratory (ORNL) in Oak Ridge, Tennessee, United States. Operating at 85 MW, HFIR is one of the highest flux reactor-based sources of neutrons for cond ...
(HFIR) at the Oak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a U.S. multiprogram science and technology national laboratory sponsored by the U.S. Department of Energy (DOE) and administered, managed, and operated by UT–Battelle as a federally funded research an ...
in Tennessee
Tennessee ( , ), officially the State of Tennessee, is a landlocked U.S. state, state in the Southeastern United States, Southeastern region of the United States. Tennessee is the List of U.S. states and territories by area, 36th-largest by ...
, USA, which is dedicated to the production of transcurium (''Z'' > 96) elements. Lower mass fermium isotopes are available in greater quantities, though these isotopes (254Fm and 255Fm) are comparatively short-lived. In a "typical processing campaign" at Oak Ridge, tens of grams of curium
Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first in ...
are irradiated to produce decigram quantities of californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding c ...
, milligram quantities of berkelium
Berkelium is a transuranic radioactive chemical element with the symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence B ...
and einsteinium
Einsteinium is a synthetic element with the symbol Es and atomic number 99. Einsteinium is a member of the actinide series and it is the seventh transuranium element. It was named in honor of Albert Einstein.
Einsteinium was discovered as a com ...
, and picogram quantities of fermium. However, nanogram quantities of fermium can be prepared for specific experiments. The quantities of fermium produced in 20–200 kiloton thermonuclear explosions is believed to be of the order of milligrams, although it is mixed in with a huge quantity of debris; 4.0 picograms of 257Fm was recovered from 10 kilograms of debris from the " Hutch" test (16 July 1969). The Hutch experiment produced an estimated total of 250 micrograms of 257Fm.
After production, the fermium must be separated from other actinides and from lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yt ...
fission products. This is usually achieved by ion-exchange chromatography
Ion chromatography (or ion-exchange chromatography) separates ions and polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including large proteins, small nucleotides, and amino a ...
, with the standard process using a cation exchanger such as Dowex 50 or TEVA eluted with a solution of ammonium α-hydroxyisobutyrate. Smaller cations form more stable complexes with the α-hydroxyisobutyrate anion, and so are preferentially eluted from the column. A rapid fractional crystallization method has also been described.
Although the most stable isotope of fermium is 257Fm, with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of 100.5 days, most studies are conducted on 255Fm (''t''1/2 = 20.07(7) hours), since this isotope can be easily isolated as required as the decay product of 255Es (''t''1/2 = 39.8(12) days).
Synthesis in nuclear explosions
The analysis of the debris at the 10- megaton ''Ivy Mike'' nuclear test was a part of long-term project, one of the goals of which was studying the efficiency of production of transuranium elements in high-power nuclear explosions. The motivation for these experiments was as follows: synthesis of such elements from uranium requires multiple neutron capture. The probability of such events increases with the neutron flux, and nuclear explosions are the most powerful neutron sources, providing densities of the order 1023 neutrons/cm2 within a microsecond, i.e. about 1029 neutrons/(cm2·s). In comparison, the flux of the HFIR reactor is 5 neutrons/(cm2·s). A dedicated laboratory was set up right atEnewetak Atoll
Enewetak Atoll (; also spelled Eniwetok Atoll or sometimes Eniewetok; mh, Ānewetak, , or , ; known to the Japanese as Brown Atoll or Brown Island; ja, ブラウン環礁) is a large coral atoll of 40 islands in the Pacific Ocean and with it ...
for preliminary analysis of debris, as some isotopes could have decayed by the time the debris samples reached the U.S. The laboratory was receiving samples for analysis, as soon as possible, from airplanes equipped with paper filters which flew over the atoll after the tests. Whereas it was hoped to discover new chemical elements heavier than fermium, those were not found after a series of megaton explosions conducted between 1954 and 1956 at the atoll.Seaborg, p. 39
 The atmospheric results were supplemented by the underground test data accumulated in the 1960s at the
The atmospheric results were supplemented by the underground test data accumulated in the 1960s at the Nevada Test Site
The Nevada National Security Site (N2S2 or NNSS), known as the Nevada Test Site (NTS) until 2010, is a United States Department of Energy (DOE) reservation located in southeastern Nye County, Nevada, about 65 miles (105 km) northwest of the ...
, as it was hoped that powerful explosions conducted in confined space might result in improved yields and heavier isotopes. Apart from traditional uranium charges, combinations of uranium with americium and thorium have been tried, as well as a mixed plutonium-neptunium charge. They were less successful in terms of yield, which was attributed to stronger losses of heavy isotopes due to enhanced fission rates in heavy-element charges. Isolation of the products was found to be rather problematic, as the explosions were spreading debris through melting and vaporizing rocks under the great depth of 300–600 meters, and drilling to such depth in order to extract the products was both slow and inefficient in terms of collected volumes.Seaborg, p. 40
Among the nine underground tests, which were carried between 1962 and 1969 and codenamed Anacostia (5.2 kilotons
TNT equivalent is a convention for expressing energy, typically used to describe the energy released in an explosion. The is a unit of energy defined by that convention to be , which is the approximate energy released in the detonation of a ...
, 1962), Kennebec (<5 kilotons, 1963), Par (38 kilotons, 1964), Barbel (<20 kilotons, 1964), Tweed (<20 kilotons, 1965), Cyclamen (13 kilotons, 1966), Kankakee (20-200 kilotons, 1966), Vulcan (25 kilotons, 1966) and Hutch (20-200 kilotons, 1969), the last one was most powerful and had the highest yield of transuranium elements. In the dependence on the atomic mass number, the yield showed a saw-tooth behavior with the lower values for odd isotopes, due to their higher fission rates. The major practical problem of the entire proposal, however, was collecting the radioactive debris dispersed by the powerful blast. Aircraft filters adsorbed only about 4 of the total amount and collection of tons of corals at Enewetak Atoll increased this fraction by only two orders of magnitude. Extraction of about 500 kilograms of underground rocks 60 days after the Hutch explosion recovered only about 10−7 of the total charge. The amount of transuranium elements in this 500-kg batch was only 30 times higher than in a 0.4 kg rock picked up 7 days after the test. This observation demonstrated the highly nonlinear dependence of the transuranium elements yield on the amount of retrieved radioactive rock.Seaborg, p. 43 In order to accelerate sample collection after explosion, shafts were drilled at the site not after but before the test, so that explosion would expel radioactive material from the epicenter, through the shafts, to collecting volumes near the surface. This method was tried in the Anacostia and Kennebec tests and instantly provided hundreds kilograms of material, but with actinide concentration 3 times lower than in samples obtained after drilling; whereas such method could have been efficient in scientific studies of short-lived isotopes, it could not improve the overall collection efficiency of the produced actinides.Seaborg, p. 44
Although no new elements (apart from einsteinium and fermium) could be detected in the nuclear test debris, and the total yields of transuranium elements were disappointingly low, these tests did provide significantly higher amounts of rare heavy isotopes than previously available in laboratories. For example, 6 atoms of 257Fm could be recovered after the Hutch detonation. They were then used in the studies of thermal-neutron induced fission of 257Fm and in discovery of a new fermium isotope 258Fm. Also, the rare 250Cm isotope was synthesized in large quantities, which is very difficult to produce in nuclear reactors from its progenitor 249Cm; the half-life of 249Cm (64 minutes) is much too short for months-long reactor irradiations, but is very "long" on the explosion timescale.Seaborg, p. 47
Natural occurrence
Because of the short half-life of all isotopes of fermium, anyprimordial
Primordial may refer to:
* Primordial era, an era after the Big Bang. See Chronology of the universe
* Primordial sea (a.k.a. primordial ocean, ooze or soup). See Abiogenesis
* Primordial nuclide, nuclides, a few radioactive, that formed before t ...
fermium, that is fermium that could be present on the Earth during its formation, has decayed by now. Synthesis of fermium from naturally occurring actinides uranium and thorium in the Earth crust requires multiple neutron capture, which is an extremely unlikely event. Therefore, most fermium is produced on Earth in scientific laboratories, high-power nuclear reactors, or in nuclear weapons tests
Nuclear weapons tests are experiments carried out to determine nuclear weapons' effectiveness, yield, and explosive capability. Testing nuclear weapons offers practical information about how the weapons function, how detonations are affected b ...
, and is present only within a few months from the time of the synthesis. The transuranic elements from americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was n ...
to fermium did occur naturally in the natural nuclear fission reactor
A natural nuclear fission reactor is a uranium deposit where self-sustaining nuclear chain reactions occur. The conditions under which a natural nuclear reactor could exist had been predicted in 1956 by Japanese American chemist Paul Kuroda. ...
at Oklo
Oklo is a region near the town of Franceville, in the Haut-Ogooué province of the Central African country of Gabon. Several natural nuclear fission reactors were discovered in the uranium mines in the region in 1972.
History
Gabon was a Frenc ...
, but no longer do so.
Chemistry
 The chemistry of fermium has only been studied in solution using tracer techniques, and no solid compounds have been prepared. Under normal conditions, fermium exists in solution as the Fm3+ ion, which has a
The chemistry of fermium has only been studied in solution using tracer techniques, and no solid compounds have been prepared. Under normal conditions, fermium exists in solution as the Fm3+ ion, which has a hydration number
The hydration number, or solvation number of a compound is defined as the average number of molecules bound to the compound more strongly (by 13.3 kcal/mol or more) than they are bound to other water molecules. The hydration number is dependent o ...
of 16.9 and an acid dissociation constant
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted ) is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction
:HA ...
of 1.6 (p''K''a = 3.8). Fm3+ forms complexes with a wide variety of organic ligands with hard donor atoms such as oxygen, and these complexes are usually more stable than those of the preceding actinides. It also forms anionic complexes with ligands such as chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride s ...
or nitrate
Nitrate is a polyatomic ion with the chemical formula . Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insolu ...
and, again, these complexes appear to be more stable than those formed by einsteinium
Einsteinium is a synthetic element with the symbol Es and atomic number 99. Einsteinium is a member of the actinide series and it is the seventh transuranium element. It was named in honor of Albert Einstein.
Einsteinium was discovered as a com ...
or californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding c ...
. It is believed that the bonding in the complexes of the later actinides is mostly ionic in character: the Fm3+ ion is expected to be smaller than the preceding An3+ ions because of the higher effective nuclear charge In atomic physics, the effective nuclear charge is the actual amount of positive (nuclear) charge experienced by an electron in a multi-electron atom. The term "effective" is used because the shielding effect of negatively charged electrons prevent ...
of fermium, and hence fermium would be expected to form shorter and stronger metal–ligand bonds.
Fermium(III) can be fairly easily reduced to fermium(II), for example with samarium(II) chloride
Samarium(II) chloride ( Sm Cl2) is a chemical compound, used as a radical generating agent in the ketone-mediated intraannulation reaction.
Preparation
Reduction of samarium(III) chloride with samarium metal in a vacuum at a temperature of 800&nb ...
, with which fermium(II) coprecipitates. In the precipitate, the compound fermium(II) chloride (FmCl2) was produced, though it was not purified or studied in isolation. The electrode potential
In electrochemistry, electrode potential is the electromotive force of a galvanic cell built from a standard reference electrode and another electrode to be characterized. By convention, the reference electrode is the standard hydrogen electrode ...
has been estimated to be similar to that of the ytterbium
Ytterbium is a chemical element with the symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. However, like the othe ...
(III)/(II) couple, or about −1.15 V with respect to the standard hydrogen electrode
The standard hydrogen electrode (abbreviated SHE), is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials. Its absolute electrode potential is estimated to be at 25 °C, but to form a basis ...
, a value which agrees with theoretical calculations. The Fm2+/Fm0 couple has an electrode potential of −2.37(10) V based on polarographic measurements.
Toxicity
Although few people come in contact with fermium, theInternational Commission on Radiological Protection
The International Commission on Radiological Protection (ICRP) is an independent, international, non-governmental organization, with the mission to protect people, animals, and the environment from the harmful effects of ionising radiation. Its r ...
has set annual exposure limits for the two most stable isotopes. For fermium-253, the ingestion limit was set at 107 becquerel
The becquerel (; symbol: Bq) is the unit of radioactivity in the International System of Units (SI). One becquerel is defined as the activity of a quantity of radioactive material in which one nucleus decays per second. For applications relatin ...
s (1 Bq is equivalent to one decay per second), and the inhalation limit at 105 Bq; for fermium-257, at 105 Bq and 4000 Bq respectively.
Notes and references
Notes
References
Further reading
* Robert J. SilvaFermium, Mendelevium, Nobelium, and Lawrencium
in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Hrsg.): ''The Chemistry of the Actinide and Transactinide Elements'', Springer, Dordrecht 2006; , p. 1621–1651; . * Seaborg, Glenn T. (ed.) (1978)
Proceedings of the Symposium Commemorating the 25th Anniversary of Elements 99 and 100
', 23 January 1978, Report LBL-7701 * '' Gmelins Handbuch der anorganischen Chemie'', System Nr. 71, Transurane: Teil A 1 II, p. 19–20; Teil A 2, p. 47; Teil B 1, p. 84.
External links
Fermium
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
{{Authority control
Chemical elements
Chemical elements with face-centered cubic structure
Actinides
Synthetic elements
Enrico Fermi