electrophile on:
[Wikipedia]
[Google]
[Amazon]
In
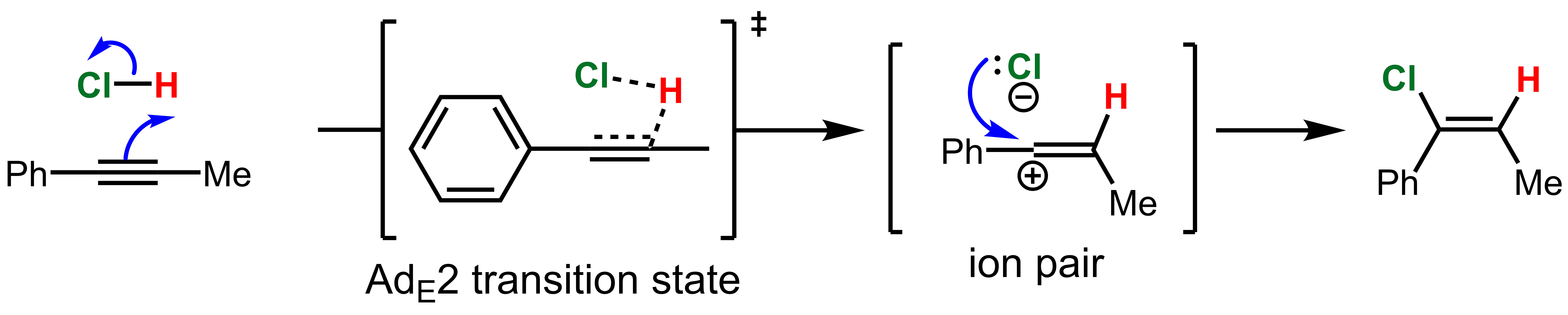
 In contrast, phenylpropyne reacts by the AdE2ip ("addition, electrophilic, second-order, ion pair") mechanism to give predominantly the ''syn'' product (~10:1 ''syn'':''anti''). In this case, the intermediate vinyl cation is formed by addition of HCl because it is resonance-stabilized by the phenyl group. Nevertheless, the lifetime of this high energy species is short, and the resulting vinyl cation-chloride anion ion pair immediately collapses, before the chloride ion has a chance to leave the solvent shell, to give the vinyl chloride. The proximity of the anion to the side of the vinyl cation where the proton was added is used to rationalize the observed predominance of ''syn'' addition.
In contrast, phenylpropyne reacts by the AdE2ip ("addition, electrophilic, second-order, ion pair") mechanism to give predominantly the ''syn'' product (~10:1 ''syn'':''anti''). In this case, the intermediate vinyl cation is formed by addition of HCl because it is resonance-stabilized by the phenyl group. Nevertheless, the lifetime of this high energy species is short, and the resulting vinyl cation-chloride anion ion pair immediately collapses, before the chloride ion has a chance to leave the solvent shell, to give the vinyl chloride. The proximity of the anion to the side of the vinyl cation where the proton was added is used to rationalize the observed predominance of ''syn'' addition.
 Oxaziridines such as chiral N-sulfonyloxaziridines effect enantioselective ketone alpha oxidation en route to the AB-ring segments of various
Oxaziridines such as chiral N-sulfonyloxaziridines effect enantioselective ketone alpha oxidation en route to the AB-ring segments of various
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, an electrophile is a chemical species
Chemical species are a specific form of chemical substance or chemically identical molecular entities that have the same molecular energy level at a specified timescale. These entities are classified through bonding types and relative abundance of ...
that forms bonds with nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
s by accepting an electron pair
In chemistry, an electron pair or Lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Gilbert N. Lewis introduced the concepts of both the electron pair and the covalent bond in a landmark paper ...
. Because electrophiles accept electrons, they are Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
s. Most electrophiles are positively charge
Charge or charged may refer to:
Arts, entertainment, and media Films
* ''Charge, Zero Emissions/Maximum Speed'', a 2011 documentary
Music
* ''Charge'' (David Ford album)
* ''Charge'' (Machel Montano album)
* '' Charge!!'', an album by The Aqu ...
d, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons.
Electrophiles mainly interact with nucleophiles through addition
Addition (usually signified by the Plus and minus signs#Plus sign, plus symbol, +) is one of the four basic Operation (mathematics), operations of arithmetic, the other three being subtraction, multiplication, and Division (mathematics), divis ...
and substitution reactions. Frequently seen electrophiles in organic syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and expe ...
include cations
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
such as H+ and NO+, polarized neutral molecules such as HCl, alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
s, acyl halides, and carbonyl compound
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such as aldehydes, ...
s, polarizable neutral molecules such as Cl2 and Br2, oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ''electron donor''). In ot ...
s such as organic peracid
A peroxy acid (often spelled as one word, peroxyacid, and sometimes called peracid) is an acid which contains an acidic group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the peroxy deri ...
s, chemical species that do not satisfy the octet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The ru ...
such as carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
s and radicals
Radical (from Latin: ', root) may refer to:
Politics and ideology Politics
*Classical radicalism, the Radical Movement that began in late 18th century Britain and spread to continental Europe and Latin America in the 19th century
*Radical politics ...
, and some Lewis acids such as BH3 and DIBAL.
Organic chemistry
Addition of halogens
These occur between alkenes and electrophiles, often halogens as inhalogen addition reaction A halogen addition reaction is a simple organic reaction where a halogen molecule is added to the carbon–carbon double bond of an alkene functional group.
The general chemical formula of the halogen addition reaction is:
:C=C + X2 → X−C−C ...
s. Common reactions include use of bromine water to titrate
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of Quantitative research, quantitative Analytical chemistry, chemical analysis to determine the concentration of an identified analyte (a substance to be ...
against a sample to deduce the number of double bonds present. For example, ethene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon double bonds).
Ethy ...
+ bromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
→ 1,2-dibromoethane
1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula . Although trace amounts occur naturally in the ocean, where it is probably formed by algae and kelp, substantial amounts are produc ...
:
:C2H4 + Br2 → BrCH2CH2Br
This takes the form of 3 main steps shown below;
:
# Forming of a π-complex
#: The electrophilic Br-Br molecule interacts with electron-rich alkene molecule to form a π-complex 1.
# Forming of a three-membered bromonium ion
#: The alkene is working as an electron donor and bromine as an electrophile. The three-membered bromonium ion 2 consisted of two carbon atoms and a bromine atom forms with a release of Br−.
# Attacking of bromide ion
#: The bromonium ion is opened by the attack of Br− from the back side. This yields the vicinal dibromide with an antiperiplanar configuration. When other nucleophiles such as water or alcohol are existing, these may attack 2 to give an alcohol or an ether.
This process is called AdE2 mechanism ("addition, electrophilic, second-order"). Iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
(I2), chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
(Cl2), sulfenyl ion (RS+), mercury cation (Hg2+), and dichlorocarbene
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapi ...
(:CCl2) also react through similar pathways. The direct conversion of 1 to 3 will appear when the Br− is large excess in the reaction medium. A β-bromo carbenium ion
The carbenium ion is a kind of cation, positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. Carbenium ions are a major subset of carbocations, whic ...
intermediate may be predominant instead of 3 if the alkene has a cation-stabilizing substituent like phenyl group. There is an example of the isolation of the bromonium ion 2.
Addition of hydrogen halides
Hydrogen halides such as hydrogen chloride (HCl) adds to alkenes to give alkyl halides inhydrohalogenation
A hydrohalogenation reaction is the electrophilic addition of hydrogen halides like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes.
:
If the two carbon atoms at the double bond are linked to a different ...
. For example, the reaction of HCl with ethylene furnishes chloroethane. The reaction proceeds with a cation intermediate, being different from the above halogen addition. An example is shown below:
:
# Proton (H+) adds (by working as an electrophile) to one of the carbon atoms on the alkene to form cation 1.
# Chloride ion (Cl−) combines with the cation 1 to form the adducts 2 and 3.
In this manner, the stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation ...
of the product, that is, from which side Cl− will attack relies on the types of alkenes applied and conditions of the reaction. At least, which of the two carbon atoms will be attacked by H+ is usually decided by Markovnikov's rule
In organic chemistry, Markovnikov's rule or Markownikoff's rule describes the outcome of some addition reactions. The rule was formulated by Russian chemist Vladimir Markovnikov in 1870.
Explanation
The rule states that with the addition of a ...
. Thus, H+ attacks the carbon atom that carries fewer substituents so as the more stabilized carbocation (with the more stabilizing substituents) will form.
This is another example of an AdE2 mechanism. Hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
(HF) and hydrogen iodide (HI) react with alkenes in a similar manner, and Markovnikov-type products will be given. Hydrogen bromide
Hydrogen bromide is the inorganic compound with the formula . It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temper ...
(HBr) also takes this pathway, but sometimes a radical process competes and a mixture of isomers may form. Although introductory textbooks seldom mentions this alternative, the AdE2 mechanism is generally competitive with the AdE3 mechanism (described in more detail for alkynes, below), in which transfer of the proton and nucleophilic addition occur in a concerted manner. The extent to which each pathway contributes depends on the several factors like the nature of the solvent (e.g., polarity), nucleophilicity of the halide ion, stability of the carbocation, and steric effects. As brief examples, the formation of a sterically unencumbered, stabilized carbocation favors the AdE2 pathway, while a more nucleophilic bromide ion favors the AdE3 pathway to a greater extent compared to reactions involving the chloride ion.
In the case of dialkyl-substituted alkynes (e.g., 3-hexyne), the intermediate vinyl cation that would result from this process is highly unstable. In such cases, the simultaneous protonation (by HCl) and attack of the alkyne by the nucleophile (Cl−) is believed to take place. This mechanistic pathway is known by the Ingold label AdE3 ("addition, electrophilic, third-order"). Because the simultaneous collision of three chemical species in a reactive orientation is improbable, the termolecular transition state is believed to be reached when the nucleophile attacks a reversibly-formed weak association of the alkyne and HCl. Such a mechanism is consistent with the predominantly ''anti'' addition (>15:1 ''anti'':''syn'' for the example shown) of the hydrochlorination product and the termolecular rate law, Rate = ''k'' lkyne Clsup>2. In support of the proposed alkyne-HCl association, a T-shaped complex of an alkyne and HCl has been characterized crystallographically.
 In contrast, phenylpropyne reacts by the AdE2ip ("addition, electrophilic, second-order, ion pair") mechanism to give predominantly the ''syn'' product (~10:1 ''syn'':''anti''). In this case, the intermediate vinyl cation is formed by addition of HCl because it is resonance-stabilized by the phenyl group. Nevertheless, the lifetime of this high energy species is short, and the resulting vinyl cation-chloride anion ion pair immediately collapses, before the chloride ion has a chance to leave the solvent shell, to give the vinyl chloride. The proximity of the anion to the side of the vinyl cation where the proton was added is used to rationalize the observed predominance of ''syn'' addition.
In contrast, phenylpropyne reacts by the AdE2ip ("addition, electrophilic, second-order, ion pair") mechanism to give predominantly the ''syn'' product (~10:1 ''syn'':''anti''). In this case, the intermediate vinyl cation is formed by addition of HCl because it is resonance-stabilized by the phenyl group. Nevertheless, the lifetime of this high energy species is short, and the resulting vinyl cation-chloride anion ion pair immediately collapses, before the chloride ion has a chance to leave the solvent shell, to give the vinyl chloride. The proximity of the anion to the side of the vinyl cation where the proton was added is used to rationalize the observed predominance of ''syn'' addition.

Hydration
One of the more complexhydration reaction
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed indust ...
s utilises sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
as a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
. This reaction occurs in a similar way to the addition reaction but has an extra step in which the OSO3H group is replaced by an OH group, forming an alcohol:
:C2H4 + H2O → C2H5OH
As can be seen, the H2SO4 does take part in the overall reaction, however it remains unchanged so is classified as a catalyst.
This is the reaction in more detail:
:
# The H–OSO3H molecule has a δ+ charge on the initial H atom. This is attracted to and reacts with the double bond in the same way as before.
# The remaining (negatively charged) −OSO3H ion then attaches to the carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
, forming ethyl hydrogensulphate (upper way on the above scheme).
# When water (H2O) is added and the mixture heated, ethanol (C2H5OH) is produced. The "spare" hydrogen atom from the water goes into "replacing" the "lost" hydrogen and, thus, reproduces sulfuric acid. Another pathway in which water molecule combines directly to the intermediate carbocation (lower way) is also possible. This pathway become predominant when aqueous sulfuric acid is used.
Overall, this process adds a molecule of water to a molecule of ethene.
This is an important reaction in industry, as it produces ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
, whose purposes include fuels and starting material for other chemicals.
Chiral derivatives
Many electrophiles are chiral and optically stable. Typically chiral electrophiles are also optically pure. One suchreagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
is the fructose
Fructose (), or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and gal ...
-derived organocatalyst used in the Shi epoxidation. The catalyst can accomplish highly enantioselective epoxidations of ''trans''-disubstituted and trisubstituted alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
s. The Shi catalyst, a ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
, is oxidized by stoichiometric oxone
Potassium peroxymonosulfate is widely used as an oxidizing agent, for example, in pools and spas (usually referred to as monopersulfate or "MPS"). It is the potassium salt of peroxymonosulfuric acid. Potassium peroxymonosulfate per se is rarely e ...
to the active dioxirane
In chemistry, dioxirane (systematically named dioxacyclopropane, also known as methylene peroxide or peroxymethane) is an organic compound with formula . The molecule consists of a ring with one methylene and two oxygen atoms. It is of interes ...
form before proceeding in the catalytic cycle.
 Oxaziridines such as chiral N-sulfonyloxaziridines effect enantioselective ketone alpha oxidation en route to the AB-ring segments of various
Oxaziridines such as chiral N-sulfonyloxaziridines effect enantioselective ketone alpha oxidation en route to the AB-ring segments of various natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
s, including γ-rhodomycionone and α-citromycinone.
Polymer-bound chiral selenium
Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elem ...
electrophiles effect asymmetric selenenylation reactions. The reagents are aryl selenenyl bromides, and they were first developed for solution phase chemistry and then modified for solid phase bead attachment via an aryloxy moiety. The solid-phase reagents were applied toward the selenenylation of various alkenes with good enantioselectivities. The products can be cleaved from the solid support using organotin
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide (), discove ...
hydride reducing agents. Solid-supported reagents offers advantages over solution phase chemistry due to the ease of workup and purification.
Electrophilicity scale
Several methods exist to rank electrophiles in order of reactivity and one of them is devised by Robert Parr with the electrophilicity index ω given as: : with theelectronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
and chemical hardness. This equation is related to the classical equation for electrical power
Electric power is the rate of transfer of electrical energy within a electric circuit, circuit. Its SI unit is the watt, the general unit of power (physics), power, defined as one joule per second. Standard prefixes apply to watts as with oth ...
:
:
where is the resistance (Ohm
Ohm (symbol Ω) is a unit of electrical resistance named after Georg Ohm.
Ohm or OHM may also refer to:
People
* Georg Ohm (1789–1854), German physicist and namesake of the term ''ohm''
* Germán Ohm (born 1936), Mexican boxer
* Jörg Ohm (1 ...
or Ω) and is voltage
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a Electrostatics, static electric field, it corresponds to the Work (electrical), ...
. In this sense the electrophilicity index is a kind of electrophilic power. Correlations have been found between electrophilicity of various chemical compounds and reaction rates in biochemical systems and such phenomena as allergic contact dermititis.
An electrophilicity index also exists for free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
s. Strongly electrophilic radicals such as the halogens react with electron-rich reaction sites, and strongly nucleophilic radicals such as the 2-hydroxypropyl-2-yl and tert-butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, giv ...
radical react with a preference for electron-poor reaction sites.
Superelectrophiles
Superelectrophiles are defined as cationic electrophilic reagents with greatly enhanced reactivities in the presence ofsuperacid
In chemistry, a superacid (according to the original definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superacid i ...
s. These compounds were first described by George A. Olah. Superelectrophiles form as a doubly electron deficient superelectrophile by protosolvation of a cationic electrophile. As observed by Olah, a mixture of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
and boron trifluoride
Boron trifluoride is the inorganic compound with the formula . This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bonding
The g ...
is able to remove a hydride ion from isobutane
Isobutane, also known as ''i''-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH3)3. It is an isomer of butane. Isobutane is a colorless, odorless gas.
It is the simplest alkane with a tertiary carbon a ...
when combined with hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling p ...
via the formation of a superacid
In chemistry, a superacid (according to the original definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (), which has a Hammett acidity function (''H''0) of −12. According to the modern definition, a superacid i ...
from BF3 and HF. The responsible reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
is the H3CO2H3sup>2+ dication. Likewise, methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
can be nitrated to nitromethane
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula . It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in ...
with nitronium tetrafluoroborate
Nitronium tetrafluoroborate is an inorganic compound with formula NO2BF4. It is a salt of nitronium cation and tetrafluoroborate anion. It is a colorless crystalline solid, which reacts with water to form the corrosive acids HF and HNO3. As su ...
NOBF only in presence of a strong acid like fluorosulfuric acid
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula . It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, , substitu ...
via the protonated nitronium dication.
In gitionic (gitonic) superelectrophiles, charged centers are separated by no more than one atom, for example, the protonitronium ion O=N+=O+—H (a protonated nitronium ion
The nitronium ion, , is a cation. It is an onium ion because its nitrogen atom has +1 charge, similar to ammonium ion . It is created by the removal of an electron from the paramagnetic nitrogen dioxide molecule , or the protonation of nitri ...
). And, in distonic superelectrophiles, they are separated by 2 or more atoms, for example, in the fluorination reagent F-TEDA-BF4.
See also
*Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
* TRPA1
Transient receptor potential cation channel, subfamily A, member 1, also known as transient receptor potential ankyrin 1, TRPA1, or The Mustard and Wasabi Receptor, is a protein that in humans is encoded by the ''TRPA1'' (and in mice and rats by ...
, the sensory neural target for electrophilic irritants in mammals.
References
{{reflist, 30em Physical organic chemistry