Electron Diffraction on:
[Wikipedia]
[Google]
[Amazon]
Electron diffraction refers to the bending of electron beams around
 Due to the diffraction, part of the electrons is
Due to the diffraction, part of the electrons is
 Character of the resulting diffraction pattern depends on whether the beam is diffracted by one single crystal or by number of differently oriented crystallites for instance in a polycrystalline material. The single-crystalline diffractogram depicts a regular pattern of bright spots. This pattern can be seen as a two-dimensional projection of reciprocal crystal lattice. If there are more contributing crystallites, the diffraction image becomes a superposition of individual crystals' diffraction patterns. Ultimately, this superposition contains diffraction spots of all possible crystallographic plane systems in all possible orientations. These conditions result in a diffractogram of
Character of the resulting diffraction pattern depends on whether the beam is diffracted by one single crystal or by number of differently oriented crystallites for instance in a polycrystalline material. The single-crystalline diffractogram depicts a regular pattern of bright spots. This pattern can be seen as a two-dimensional projection of reciprocal crystal lattice. If there are more contributing crystallites, the diffraction image becomes a superposition of individual crystals' diffraction patterns. Ultimately, this superposition contains diffraction spots of all possible crystallographic plane systems in all possible orientations. These conditions result in a diffractogram of
 Kikuchi lines are linear diffractogram features created by electrons scattered first inelastically and then elastically. As the electron beam interacts with matter, some electrons are diffracted via elastic scattering, while other can be scattered inelastically losing part of their kinetic energy. Direction of inelastically scattered electrons is significantly less predictive - they can more or less follow the direction of the original beam as well as be "reflected" back. In all cases, they continue to interact with the matter and some of them are diffracted. These electrons then form Kikuchi lines.
Kikuchi lines are paired forming Kikuchi bands. They are indexed after the crystallographic planes they were formed with. Angular width of the band is equal to the diffraction angle specified above which means, that in the diffractogram, the width of plane will be equal to the distance between transmitted beam and diffraction spot. The position of Kikuchi bands is fixed against each other but not against the diffraction spots. As the crystal is tilted in the electron beam, the bands move on the diffraction pattern.
Kikuchi lines are linear diffractogram features created by electrons scattered first inelastically and then elastically. As the electron beam interacts with matter, some electrons are diffracted via elastic scattering, while other can be scattered inelastically losing part of their kinetic energy. Direction of inelastically scattered electrons is significantly less predictive - they can more or less follow the direction of the original beam as well as be "reflected" back. In all cases, they continue to interact with the matter and some of them are diffracted. These electrons then form Kikuchi lines.
Kikuchi lines are paired forming Kikuchi bands. They are indexed after the crystallographic planes they were formed with. Angular width of the band is equal to the diffraction angle specified above which means, that in the diffractogram, the width of plane will be equal to the distance between transmitted beam and diffraction spot. The position of Kikuchi bands is fixed against each other but not against the diffraction spots. As the crystal is tilted in the electron beam, the bands move on the diffraction pattern.
 Electron diffraction in a
Electron diffraction in a
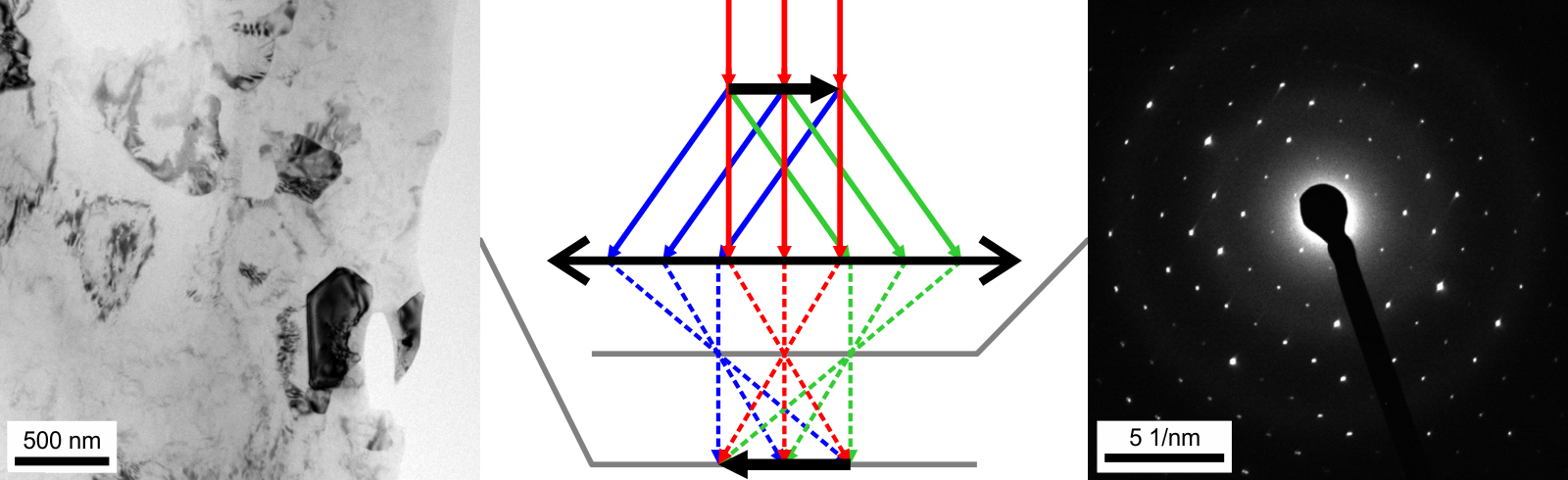 In TEM, electron beam passes through a thin film of the examined material. Before and after its interaction with the sample, the beam is formed by various elements of electron optics including
In TEM, electron beam passes through a thin film of the examined material. Before and after its interaction with the sample, the beam is formed by various elements of electron optics including
 If the sample is tilted against the electron beam, diffraction conditions are satisfied for different set of crystallographic planes yielding different constellation of diffraction spots. This allows to determine the crystal orientation, which can be used for instance to set the orientation needed for particular experiment, to determine misorientation between adjacent grains or crystal twins. Since different sample orientations result in different projections of the reciprocal lattice, they provide an opportunity to reconstruct the three-dimensional information about the crystal structure lost in individual projections. A series of diffractograms varying in tilt can be acquired and processed using a diffraction tomography analysis in order to reconstruct an unknown crystal structure.
Kikuchi lines occur in TEM especially in thicker samples, where inelastic scattering followed by the elastic one occurs with sufficient frequency. Since the position of Kikuchi bands within the diffractogram is quite sensitive to crystal orientation, they can be used to fine-tune a zone-axis orientation. Alternatively, they allow to determine crystal orientation with significantly higher accuracy than what is feasible with a spot diffraction analysis. Since mutual positions and orientations of Kikuchi bands is fixed and since the bands intersect in low-index zone axes, they can be used for navigation when changing the orientation between zone axes connected by some band. For those purposes, Kikuchi maps are available.
If the illuminated area selected by the aperture covers many differently oriented crystallites, their diffraction patterns superimpose forming an image of concentric rings. The ring diffractogram is typical for polycrystalline samples, powders or nanoparticles. A diameter of each ring corresponds to interplanar distance of a plane system present in the sample. Instead of information about individual grains or sample orientation, this diffractogram provides more of a statistical information for instance about overall crystallinity or texture. Textured materials can be recognized by a non-uniform distribution of intensity along the ring circumference despite sufficient crystallinity. Ring diffractograms can be also used to discriminate between nanocrystalline and amorphous phases.
If the sample is tilted against the electron beam, diffraction conditions are satisfied for different set of crystallographic planes yielding different constellation of diffraction spots. This allows to determine the crystal orientation, which can be used for instance to set the orientation needed for particular experiment, to determine misorientation between adjacent grains or crystal twins. Since different sample orientations result in different projections of the reciprocal lattice, they provide an opportunity to reconstruct the three-dimensional information about the crystal structure lost in individual projections. A series of diffractograms varying in tilt can be acquired and processed using a diffraction tomography analysis in order to reconstruct an unknown crystal structure.
Kikuchi lines occur in TEM especially in thicker samples, where inelastic scattering followed by the elastic one occurs with sufficient frequency. Since the position of Kikuchi bands within the diffractogram is quite sensitive to crystal orientation, they can be used to fine-tune a zone-axis orientation. Alternatively, they allow to determine crystal orientation with significantly higher accuracy than what is feasible with a spot diffraction analysis. Since mutual positions and orientations of Kikuchi bands is fixed and since the bands intersect in low-index zone axes, they can be used for navigation when changing the orientation between zone axes connected by some band. For those purposes, Kikuchi maps are available.
If the illuminated area selected by the aperture covers many differently oriented crystallites, their diffraction patterns superimpose forming an image of concentric rings. The ring diffractogram is typical for polycrystalline samples, powders or nanoparticles. A diameter of each ring corresponds to interplanar distance of a plane system present in the sample. Instead of information about individual grains or sample orientation, this diffractogram provides more of a statistical information for instance about overall crystallinity or texture. Textured materials can be recognized by a non-uniform distribution of intensity along the ring circumference despite sufficient crystallinity. Ring diffractograms can be also used to discriminate between nanocrystalline and amorphous phases.
 Gas electron diffraction (GED) can be used to determine
Gas electron diffraction (GED) can be used to determine
 In Scanning electron microscope the sample surface is mapped using a scanning electron beam. The diffraction image is formed using an electron backscatter diffraction (EBSD). A thin layer of the sample material is penetrated by the electrons, some of which are reflected due to the interactions inside the material and are diffracted on their journey out of the sample. As result of the inelastic scattering followed by the elastic one, typical features for an EBSD image are Kikuchi lines. Since the position of Kikuchi bands is highly sensitive to the crystal orientation, EBSD data acquired using the scanning beam can be used to determine the crystal orientation at particular location on the sample surface. The data are processed by an automated software allowing to routinely generate two-dimensional orientation maps across the sample surface. As the Kikuchi lines carry information about the interplanar angles and distances and, therefore, about the crystal structure, they can be also used for a phase identification or strain analysis.
In Scanning electron microscope the sample surface is mapped using a scanning electron beam. The diffraction image is formed using an electron backscatter diffraction (EBSD). A thin layer of the sample material is penetrated by the electrons, some of which are reflected due to the interactions inside the material and are diffracted on their journey out of the sample. As result of the inelastic scattering followed by the elastic one, typical features for an EBSD image are Kikuchi lines. Since the position of Kikuchi bands is highly sensitive to the crystal orientation, EBSD data acquired using the scanning beam can be used to determine the crystal orientation at particular location on the sample surface. The data are processed by an automated software allowing to routinely generate two-dimensional orientation maps across the sample surface. As the Kikuchi lines carry information about the interplanar angles and distances and, therefore, about the crystal structure, they can be also used for a phase identification or strain analysis.
Virtual lab on electron diffraction
*
analysis of an unknown
* PTCLab-Program for calculation phase transformation crystallography with diffraction simulation, its free and open source python program https://code.google.com/p/transformation-crystallography-lab/
ronchigram.com
Web simulator for generating convergent beam diffraction of amorphous materials. {{Authority control Diffraction Electron Quantum mechanics
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, a ...
ic structures. This behaviour, typical for waves
Waves most often refers to:
* Waves, oscillations accompanied by a transfer of energy that travel through space or mass.
* Wind waves, surface waves that occur on the free surface of bodies of water.
Waves may also refer to:
Music
*Waves (ban ...
, is applicable to electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
s due to the wave–particle duality
Wave–particle duality is the concept in quantum mechanics that every particle or quantum entity may be described as either a particle or a wave. It expresses the inability of the classical physics, classical concepts "particle" or "wave" to fu ...
stating that electrons behave as both particles and waves. Since the diffracted beams interfere, they generate diffraction patterns widely used for analysis of the objects which caused the diffraction. Therefore, electron diffraction can also refer to derived experimental techniques used for material characterization
Characterization, when used in materials science, refers to the broad and general process by which a material's structure and properties are probed and measured. It is a fundamental process in the field of materials science, without which no scien ...
. This technique is similar to X-ray
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 picometers to 10 nanometers, corresponding to frequencies in the range 30&nb ...
and neutron diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material. A sample to be examined is placed in a beam of thermal or cold neutrons to ob ...
.
Electron diffraction is most frequently used in solid state physics and chemistry to study crystalline, quasi-crystalline and amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek language, Gr ...
materials using electron microscope
An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination. As the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, electron microscopes have a hi ...
s. In these instruments, electrons are accelerated by an electrostatic potential in order to gain energy and shorten their wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tr ...
. With the wavelength sufficiently short, the atomic structure acts as a diffraction grating
In optics, a diffraction grating is an optical component with a periodic structure that diffracts light into several beams travelling in different directions (i.e., different diffraction angles). The emerging coloration is a form of structur ...
generating diffraction patterns, which carry the information about the crystal orientation, lattice parameters
Lattice may refer to:
Arts and design
* Latticework, an ornamental criss-crossed framework, an arrangement of crossing laths or other thin strips of material
* Lattice (music), an organized grid model of pitch ratios
* Lattice (pastry), an orn ...
, crystal defects
A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the unit cell para ...
etc.
In transmission electron microscopy
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a ...
(TEM), the most frequent technique related to the electron diffraction is selected area diffraction allowing to measure crystal properties or reconstruct its structure. Despite the technique was considered qualitative for a long time, thanks to modern analytical software
Software is a set of computer programs and associated documentation and data. This is in contrast to hardware, from which the system is built and which actually performs the work.
At the lowest programming level, executable code consist ...
it offers quantitative
Quantitative may refer to:
* Quantitative research, scientific investigation of quantitative properties
* Quantitative analysis (disambiguation)
* Quantitative verse, a metrical system in poetry
* Statistics, also known as quantitative analysis
...
analysis. In scanning electron microscopy (SEM), electron backscatter diffraction is used to determine crystal orientation across the sample. The potential of electron diffraction is not only limited to solids. It can be used to characterize individual molecules dispersed in a gaseous atmosphere using gas electron diffraction.
History
Experiments involving electron beam are dated long before the discovery of electron. In 1650, Otto von Guericke inventedvacuum pump
A vacuum pump is a device that draws gas molecules from a sealed volume in order to leave behind a partial vacuum. The job of a vacuum pump is to generate a relative vacuum within a capacity. The first vacuum pump was invented in 1650 by Otto ...
allowing the physicists to study effects of high voltage electricity passing through rarefied air. It was noted that electrostatic generator sparks travel a longer distance through low pressure air than through atmospheric pressure air. In 1857, German physicist Heinrich Geissler was able to achieve a pressure of around 10−3 atm and with voltages between a few kilovolts and 100 kV he observed glow discharge
A glow discharge is a plasma formed by the passage of electric current through a gas. It is often created by applying a voltage between two electrodes in a glass tube containing a low-pressure gas. When the voltage exceeds a value called the st ...
. By the 1870s, British physicist William Crookes and others were able to evacuate tubes below 10−6 atm and observed, that the glow in the whole tube disappeared with lowering pressure but the glass behind the anode began to glow. The reason was, that the low pressure allowed the electrons to fly from cathode to the anode without collisions. Even though they were attracted to the anode, they flew so fast, they passed the anode and collided with the tube wall behind making it glow.
The electron beam was discovered in 1869 by German physicist Johann Hittorf. He noticed a shadow cast by the anode on the tube wall behind the anode. He correctly induced that there must be rays emitted from the cathode. Another German scientist Eugen Goldstein named them ''cathode rays'' (German ''kathodenstrahlen''). In 1897, Joseph Thomson measured the mass of cathode rays proving they were made of particles. These particles, however, were 1800 times lighter than the lightest particle known by then - a hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
atom. Therefore, the first subatomic particle
In physical sciences, a subatomic particle is a particle that composes an atom. According to the Standard Model of particle physics, a subatomic particle can be either a composite particle, which is composed of other particles (for example, a p ...
was discovered, originally called ''corpuscle'' and later named ''electron''. Thomson also showed electrons were identical with particles given off by photoelectric and radioactive materials.
Paradoxically, Thomson's discovery did not bring the cathode rays and diffraction much closer to each other, since the diffraction was a phenomenon specific to waves, not particles like electrons. The light diffraction was first described already in the 17th century by Italian priest and physicist Francesco Maria Grimaldi. But the light was suggested to be of a wave nature in 1803 when British scientist Thomas Young performed his experiment
An experiment is a procedure carried out to support or refute a hypothesis, or determine the efficacy or likelihood of something previously untried. Experiments provide insight into cause-and-effect by demonstrating what outcome occurs whe ...
with two slits. The wave theory was further supported by studies and calculations of French physicist Augustin-Jean Fresnel 1816 and 1818 finally confirming theory of Christiaan Huygens
Christiaan Huygens, Lord of Zeelhem, ( , , ; also spelled Huyghens; la, Hugenius; 14 April 1629 – 8 July 1695) was a Dutch mathematician, physicist, engineer, astronomer, and inventor, who is regarded as one of the greatest scientists o ...
.
Understanding to the nature of electron beam has fundamentally changed in 1925, when French physicist Louis de Broglie published his hypothesis
A hypothesis (plural hypotheses) is a proposed explanation for a phenomenon. For a hypothesis to be a scientific hypothesis, the scientific method requires that one can test it. Scientists generally base scientific hypotheses on previous obse ...
. He stated that all matter particles can behave as waves and therefore, among other important implications, can be diffracted. The de Broglie hypothesis was experimentally confirmed for electrons in two experiments performed independently by George Paget Thomson
Sir George Paget Thomson, FRS (; 3 May 189210 September 1975) was a British physicist and Nobel laureate in physics recognized for his discovery of the wave properties of the electron by electron diffraction.
Education and early life
Thomson ...
and Clinton Joseph Davisson. This sparked a rapid development of electron-based analytical techniques in the 1930s from gas electron diffraction invented by Herman Mark to the first electron microscopes developed by Ernst Ruska.
Theory
Electron interaction with matter
In order to be diffracted, electrons need tointeract
Advocates for Informed Choice, doing business as, dba interACT or interACT Advocates for Intersex Youth, is a 501(c)(3) nonprofit organization using innovative strategies to advocate for the legal and human rights of children with intersex trai ...
with matter
In classical physics and general chemistry, matter is any substance that has mass and takes up space by having volume. All everyday objects that can be touched are ultimately composed of atoms, which are made up of interacting subatomic part ...
. Due to their negative electric charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons res ...
, their interactions differ from other radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or through a material medium. This includes:
* ''electromagnetic radiation'', such as radio waves, microwaves, infrared, visi ...
used in diffraction studies of materials such as X-ray
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 picometers to 10 nanometers, corresponding to frequencies in the range 30&nb ...
s and neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
s. Negative electrons are scattered due to Coulomb forces when they interact with a positively charged atomic core. In comparison, X-rays are scattered after interactions with valence electrons, while neutrons are scattered by the atomic nuclei through the strong nuclear force.
Electron diffraction occurs as the result of an elastic scattering, when the incident electrons do not lose their kinetic energy
In physics, the kinetic energy of an object is the energy that it possesses due to its motion.
It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acce ...
in their interactions with atoms. In some cases, however, even inelastically scattered electrons can be diffracted as the result of a following elastic interaction.
Wavelength of electrons
Due to the wave-particle duality, electrons behave as particles as well as waves. A basic characteristic of the wave is awavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tr ...
. The electron wavelength is given by de Broglie equation as
Here is Planck's constant and is momentum
In Newtonian mechanics, momentum (more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. If is an object's mass ...
, where is the electron mass
The electron mass (symbol: ''m''e) is the mass of a stationary electron, also known as the invariant mass of the electron. It is one of the fundamental constants of physics. It has a value of about or about , which has an energy-equivalent o ...
and the velocity. Since the electrons are charged particles, they can be accelerated using electric potential
The electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as the amount of work energy needed to move a unit of electric charge from a reference point to the specific point in ...
. This allows the electrons to get accelerated to speeds, where relativistic theory needs to be applied. Therefore, there are two definitions of wavelength - non-relativistic and relativistic.
Non-relativistic theory
In a non-relativistic theory, the electrons accelerated in an electric potential gain the velocity given by the equation where is the electron rest mass, is theelementary charge
The elementary charge, usually denoted by is the electric charge carried by a single proton or, equivalently, the magnitude of the negative electric charge carried by a single electron, which has charge −1 . This elementary charge is a fundam ...
and is the speed of light
The speed of light in vacuum, commonly denoted , is a universal physical constant that is important in many areas of physics. The speed of light is exactly equal to ). According to the special theory of relativity, is the upper limit fo ...
. Substituting the momentum and velocity to the de Broglie equation we receive
Relativistic theory
In an electron microscope, the accelerating potential is usually several thousand volts, causing the electron to travel at an appreciable fraction of the speed of light. Scanning electron microscopes typically operate at an accelerating voltage of 10,000 volts (10 kV) giving an electron velocity approximately 20% of the speed of light, while a typical TEM operates at 200 kV raising the electron velocity to 70% the speed of light. Therefore,relativistic effects
Relativistic quantum chemistry combines relativistic mechanics with quantum chemistry to calculate elemental properties and structure, especially for the heavier elements of the periodic table. A prominent example is an explanation for the color of ...
need to be taken into account. The relativistic relation between energy and momentum is
:
It follows then, that the ratio between the electron mass and its rest mass (or Lorentz factor) is
:
Then the relativistic velocity is given by the equation
:
Substitution of the De Broglie equation to the above expression of energy gives
:
which leads to the final expression for the relativistic wavelength
:
The wavelength of the electrons in a 10 kV SEM is then 12.2 × 10−12 m (12.2 pm) while in a 200 kV TEM the wavelength is 2.5 pm. In comparison, the wavelength of X-rays usually used in X-ray diffraction is in the order of 100 pm (Cu Kα: λ=154 pm).
Diffraction on atomic lattice
Wavelength of the electron beam used in a typical electron microscope is sufficiently small, that crystal lattice acts as adiffraction grating
In optics, a diffraction grating is an optical component with a periodic structure that diffracts light into several beams travelling in different directions (i.e., different diffraction angles). The emerging coloration is a form of structur ...
. Therefore a diffraction pattern can be formed with beams diffracted under certain angles and intensities.
Diffraction angles
 Due to the diffraction, part of the electrons is
Due to the diffraction, part of the electrons is scattered
Scattered may refer to:
Music
* ''Scattered'' (album), a 2010 album by The Handsome Family
* "Scattered" (The Kinks song), 1993
* "Scattered", a song by Ace Young
* "Scattered", a song by Lauren Jauregui
* "Scattered", a song by Green Day from ' ...
at particular angles (diffracted beams), while others pass through the sample without changing their direction (transmitted beams). In order to determine the diffraction angles, the electron beam normally incident to the atomic lattice can be seen as a planar wave, which is re-transmitted by each atom as a spherical wave. Due to the constructive interference, the spherical waves from number of diffracted beams under angles given by the equation
:
where the integer is an order of diffraction and is the distance between atoms if we only assume one row of atoms as shown in the illustration aside. For a real-world 3D atomic structure, it would be an interplanar distance of plane with Miller indices , which needs to be oriented parallel to the electron beam.
Intensity of diffracted beams
In the kinematic theory of diffraction, the intensity of a diffracted beam corresponding to a plane withMiller indices
Miller indices form a notation system in crystallography for lattice planes in crystal (Bravais) lattices.
In particular, a family of lattice planes of a given (direct) Bravais lattice is determined by three integers ''h'', ''k'', and ''� ...
is proportional to sqare of the structure factor, which is defined as
:
where the sum is over all atoms in the unit cell, are the positional coordinates of the -th atom, and is the atomic scattering factor
In physics, the atomic form factor, or atomic scattering factor, is a measure of the scattering amplitude of a wave by an isolated atom. The atomic form factor depends on the type of scattering, which in turn depends on the nature of the incident r ...
of the -th atom.
Kinematical theory expects the electron to interact with the matter only once. For many applications, this does not pose significant limitations. For study of more complex diffraction phenomenons, dynamical
In mathematics, a dynamical system is a system in which a function describes the time dependence of a point in an ambient space. Examples include the mathematical models that describe the swinging of a clock pendulum, the flow of water in a ...
simulations using for instance Multislice or Bloch waves is needed.
Effect of crystallinity
 Character of the resulting diffraction pattern depends on whether the beam is diffracted by one single crystal or by number of differently oriented crystallites for instance in a polycrystalline material. The single-crystalline diffractogram depicts a regular pattern of bright spots. This pattern can be seen as a two-dimensional projection of reciprocal crystal lattice. If there are more contributing crystallites, the diffraction image becomes a superposition of individual crystals' diffraction patterns. Ultimately, this superposition contains diffraction spots of all possible crystallographic plane systems in all possible orientations. These conditions result in a diffractogram of
Character of the resulting diffraction pattern depends on whether the beam is diffracted by one single crystal or by number of differently oriented crystallites for instance in a polycrystalline material. The single-crystalline diffractogram depicts a regular pattern of bright spots. This pattern can be seen as a two-dimensional projection of reciprocal crystal lattice. If there are more contributing crystallites, the diffraction image becomes a superposition of individual crystals' diffraction patterns. Ultimately, this superposition contains diffraction spots of all possible crystallographic plane systems in all possible orientations. These conditions result in a diffractogram of concentric
In geometry, two or more objects are said to be concentric, coaxal, or coaxial when they share the same center or axis. Circles, regular polygons and regular polyhedra, and spheres may be concentric to one another (sharing the same center p ...
rings of discrete radii for the following reasons:
# The discreteness of ring radii is given by the fact, that there are discrete spacings between various parallel crystallographic planes in given crystal system and therefore the beams satisfying the diffraction condition can only form diffraction spots in discrete distances from the transmitted beam.
# The concentricity and ringlike shape is given by the fact, that there are all possible orientations of crystallographic planes and therefore the diffraction spots are formed all around the transmitted beam (rings centre) at the distance (ring radius) corresponding to particular crystalographic plane.
Kikuchi lines
 Kikuchi lines are linear diffractogram features created by electrons scattered first inelastically and then elastically. As the electron beam interacts with matter, some electrons are diffracted via elastic scattering, while other can be scattered inelastically losing part of their kinetic energy. Direction of inelastically scattered electrons is significantly less predictive - they can more or less follow the direction of the original beam as well as be "reflected" back. In all cases, they continue to interact with the matter and some of them are diffracted. These electrons then form Kikuchi lines.
Kikuchi lines are paired forming Kikuchi bands. They are indexed after the crystallographic planes they were formed with. Angular width of the band is equal to the diffraction angle specified above which means, that in the diffractogram, the width of plane will be equal to the distance between transmitted beam and diffraction spot. The position of Kikuchi bands is fixed against each other but not against the diffraction spots. As the crystal is tilted in the electron beam, the bands move on the diffraction pattern.
Kikuchi lines are linear diffractogram features created by electrons scattered first inelastically and then elastically. As the electron beam interacts with matter, some electrons are diffracted via elastic scattering, while other can be scattered inelastically losing part of their kinetic energy. Direction of inelastically scattered electrons is significantly less predictive - they can more or less follow the direction of the original beam as well as be "reflected" back. In all cases, they continue to interact with the matter and some of them are diffracted. These electrons then form Kikuchi lines.
Kikuchi lines are paired forming Kikuchi bands. They are indexed after the crystallographic planes they were formed with. Angular width of the band is equal to the diffraction angle specified above which means, that in the diffractogram, the width of plane will be equal to the distance between transmitted beam and diffraction spot. The position of Kikuchi bands is fixed against each other but not against the diffraction spots. As the crystal is tilted in the electron beam, the bands move on the diffraction pattern.
Diffraction by individual molecules in gases
Diffraction can be also performed on individual molecules dispersed in a gaseous atmosphere. Since the beam is dffracted by multiple molecules with all possible orientations relative to the beam, the resulting scattering pattern consists of concentric rings analogously to the diffraction cones produced by polycrystalline materials described above. In gas electron diffraction (GED) the diffraction intensities at a particular diffraction angle are described via so-called scattering variable defined as : The total intensity is then given as a sum of partial contributions: : where results from scattering by individual atoms, by pairs of atoms and by atom triplets. Intensity corresponds to the background which, unlike the previous contributions, must be determined experimentally. Intensity of atomic scattering is defined as where , is the distance between the scattering object detector, is the intensity of the primary electron beam and is the scattering amplitude of the ''i''-th atom in the molecular structure in the experiment. is the main contribution and easily obtained for known gas composition. The most valuable information is carried by the intensity of molecular scattering , as it contains information about the distance between all pairs of atoms in the molecule, whether bonded or not. It is given by formula : where is the distance between two atoms, is the mean square amplitude of vibration between the two atoms, is the anharmonicity constant and is a phase factor which is important for atomic pairs with very different nuclear charges. The summation is performed over all atom pairs. Atomic triplet intensity is negligible in most cases.Applications
In transmission electron microscope
 Electron diffraction in a
Electron diffraction in a Transmission electron microscopy
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a ...
(TEM) is a versatile technique allowing to determine a wide range of crystallographic quantities. The versatility of TEM originates from its ability to form the electron beam using complex electron optics. Good control of the beam geometry allows to perform various diffraction techniques enabling to measure the crystal lattice constants
A lattice constant or lattice parameter is one of the physical dimensions and angles that determine the geometry of the unit cells in a crystal lattice, and is proportional to the distance between atoms in the crystal. A simple cubic crystal has o ...
, study crystal defects
A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the unit cell para ...
or even to reconstruct an unknown crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pattern ...
.
Even though the diffraction analysis in TEM is a strong analytical tool itself, it can be supported by other tools available in TEM either naturally or as an optional equipment. Among other methods, TEM provides magnified sample image or even high-resolution image, chemical analysis through energy-dispersive X-ray spectroscopy, investigations of electronic structure and bonding through electron energy loss spectroscopy, and studies of the mean inner potential through electron holography
Electron holography is holography with electron waves. Dennis Gabor invented holography in 1948 when he tried to improve resolution in electron microscope. The first attempts to perform holography with electron waves were made by Haine and Mulve ...
.
Compared to another widely used material characterization technique, X-ray diffraction, TEM analysis is significantly more localized. Due to the longer radius of Ewald sphere, electron diffraction also offers constructive interference in a broader diffractogram area resulting in bright diffraction spots in higher distances from the optical axis.
Formation of diffraction image
 In TEM, electron beam passes through a thin film of the examined material. Before and after its interaction with the sample, the beam is formed by various elements of electron optics including
In TEM, electron beam passes through a thin film of the examined material. Before and after its interaction with the sample, the beam is formed by various elements of electron optics including magnetic lens
thumb
thumb
A subtype of a magnetic lens ( quadrupole magnet) in the Maier-Leibnitz laboratory, Munich
A magnetic lens is a device for the focusing or deflection of moving charged particles, such as electrons or ions, by use of the magnetic ...
es, deflectors or apertures. Optical elements above the sample are used to control the incident beam geometry allowing for a wide and parallel beam, focused nanoscopic probe or convergent cone-shaped beam. As it interacts with the sample, part of the beam is diffracted and part is transmitted through the sample without changing its direction.
Below the sample, the beam is deflected by the magnetic lens
thumb
thumb
A subtype of a magnetic lens ( quadrupole magnet) in the Maier-Leibnitz laboratory, Munich
A magnetic lens is a device for the focusing or deflection of moving charged particles, such as electrons or ions, by use of the magnetic ...
. Each set of initially parallel rays intersect at certain point in the back focal plane forming a diffraction pattern. The transmitted rays intersect right in the optical axis
An optical axis is a line along which there is some degree of rotational symmetry in an optical system such as a camera lens, microscope or telescopic sight.
The optical axis is an imaginary line that defines the path along which light pro ...
. The diffracted rays intersect at certain distance from the optical axis (corresponding to interplanar distance of the planes diffracting the beam) and under certain azimuth
An azimuth (; from ar, اَلسُّمُوت, as-sumūt, the directions) is an angular measurement in a spherical coordinate system. More specifically, it is the horizontal angle from a cardinal direction, most commonly north.
Mathematical ...
(corresponding to the orientation of the planes diffracting the beam). In case or parallel incident beam, those intersections can be seen as bright diffraction spots typical for selected area diffraction. In the image plane below the back focal plane, a magnified image of the sample is formed. Further electron optics allows to select which plane is projected to the microscope camera and, therefore, whether a magnified image or diffractogram is acquired. Modern microscopes allow to switch between the imaging and diffraction mode by pressing a single button, which makes diffraction data easily available and accessible.
Wide parallel beam
The simplest diffraction technique in TEM is selected area (electron) diffraction (SAED) for which the incident beam is wide and parallel. In order to select particular region of interest from which the diffraction should be received, a selected area aperture is used. It is located right below the sample and can be positioned so that it only allows to pass the wanted portion of the beam blocking the rest. This way, the analysis can be limited for instance to individual crystallites. If a parallel beam is used to acquire a diffraction pattern from a single-crystal, the resulting image can be seen as a two-dimensional projection of the crystal reciprocal lattice. The depicted diffraction spots correspond to individual crystallographic planes which satisfy diffraction conditions. This allows to determine interplanar distances and angles or crystal symmetry. In combination with modern automated analytical software such as CrysTBox, SAED can be used for a quantitative analysis with picometric precision. If the sample is tilted against the electron beam, diffraction conditions are satisfied for different set of crystallographic planes yielding different constellation of diffraction spots. This allows to determine the crystal orientation, which can be used for instance to set the orientation needed for particular experiment, to determine misorientation between adjacent grains or crystal twins. Since different sample orientations result in different projections of the reciprocal lattice, they provide an opportunity to reconstruct the three-dimensional information about the crystal structure lost in individual projections. A series of diffractograms varying in tilt can be acquired and processed using a diffraction tomography analysis in order to reconstruct an unknown crystal structure.
Kikuchi lines occur in TEM especially in thicker samples, where inelastic scattering followed by the elastic one occurs with sufficient frequency. Since the position of Kikuchi bands within the diffractogram is quite sensitive to crystal orientation, they can be used to fine-tune a zone-axis orientation. Alternatively, they allow to determine crystal orientation with significantly higher accuracy than what is feasible with a spot diffraction analysis. Since mutual positions and orientations of Kikuchi bands is fixed and since the bands intersect in low-index zone axes, they can be used for navigation when changing the orientation between zone axes connected by some band. For those purposes, Kikuchi maps are available.
If the illuminated area selected by the aperture covers many differently oriented crystallites, their diffraction patterns superimpose forming an image of concentric rings. The ring diffractogram is typical for polycrystalline samples, powders or nanoparticles. A diameter of each ring corresponds to interplanar distance of a plane system present in the sample. Instead of information about individual grains or sample orientation, this diffractogram provides more of a statistical information for instance about overall crystallinity or texture. Textured materials can be recognized by a non-uniform distribution of intensity along the ring circumference despite sufficient crystallinity. Ring diffractograms can be also used to discriminate between nanocrystalline and amorphous phases.
If the sample is tilted against the electron beam, diffraction conditions are satisfied for different set of crystallographic planes yielding different constellation of diffraction spots. This allows to determine the crystal orientation, which can be used for instance to set the orientation needed for particular experiment, to determine misorientation between adjacent grains or crystal twins. Since different sample orientations result in different projections of the reciprocal lattice, they provide an opportunity to reconstruct the three-dimensional information about the crystal structure lost in individual projections. A series of diffractograms varying in tilt can be acquired and processed using a diffraction tomography analysis in order to reconstruct an unknown crystal structure.
Kikuchi lines occur in TEM especially in thicker samples, where inelastic scattering followed by the elastic one occurs with sufficient frequency. Since the position of Kikuchi bands within the diffractogram is quite sensitive to crystal orientation, they can be used to fine-tune a zone-axis orientation. Alternatively, they allow to determine crystal orientation with significantly higher accuracy than what is feasible with a spot diffraction analysis. Since mutual positions and orientations of Kikuchi bands is fixed and since the bands intersect in low-index zone axes, they can be used for navigation when changing the orientation between zone axes connected by some band. For those purposes, Kikuchi maps are available.
If the illuminated area selected by the aperture covers many differently oriented crystallites, their diffraction patterns superimpose forming an image of concentric rings. The ring diffractogram is typical for polycrystalline samples, powders or nanoparticles. A diameter of each ring corresponds to interplanar distance of a plane system present in the sample. Instead of information about individual grains or sample orientation, this diffractogram provides more of a statistical information for instance about overall crystallinity or texture. Textured materials can be recognized by a non-uniform distribution of intensity along the ring circumference despite sufficient crystallinity. Ring diffractograms can be also used to discriminate between nanocrystalline and amorphous phases.
Microprobe and nanoprobe
Apart from limiting the beam using the selected area aperture, the site-selectivity can be achieved by condensing the incident beam into a narrow electron probe. This technique is called microprobe or nanoprobe diffraction or simply nanodiffraction. Probes of diameter smaller than 1 nm can be obtained. At these extremes the probe dimension comes at the price of the beam becoming nearly parallel, even though microprobe can be achieved with parallel beam. Compared to SAED, the resulting diffraction spots can be significantly broader and blurry, making a manual analysis quite inaccurate. Accurate processing is possible with automated software like CrysTBox, which is able to take into account a number of diffraction reflections across the whole pattern significantly improving accuracy and repeatability of the analysis.Convergent beam
InConvergent Beam Electron Diffraction
Convergent beam electron diffraction (CBED) is a diffraction technique where a convergent or divergent beam (conical electron beam) of electrons is used to study materials.
History
This technique was first introduced in 1939 by Kossel and Möll ...
(CBED), the incident electrons are focused in a converging cone-shaped beam with the crossover located in the sample volume. Unlike the parallel beam, the convergent beam is able to carry information from the sample volume, not just a two-dimensional projection available in SAED. With convergent beam there is also no need for the selected area aperture, as it is inherently site-selective since the beam crossover is positioned to the object plane where the sample is located.
A CBED pattern consists of disks arranged exactly the same as the spots in SAED. Intensity within the disks, however, is not uniform, but depicts various features and symmetries reflecting the sample structure. Even though the zone axis and lattice parameter analysis based on disk positions does not significantly differ from SAED, the analysis of disks content is significantly more complex. Due to a number of contributing factors, simulation based on dynamical diffraction theory is often required. With appropriate analysis, however, CBED patterns can be used for indexation of the crystal point group, space group identification, measurement of lattice parameters, thickness or strain.
The disk diameter can be controlled using the beam convergence angle. The larger is the angle, the broader the disks are depicting more features. If the angle is increased to certain level, the disks begin to overlap deteriorating the contained information. This can be solved for instance via a large angle convergent electron beam diffraction (LACBED) where the sample is moved upwards or downwards. There are applications, however, where the overlapping disks are beneficial. Ronchigram could serve as an example. It is a CBED pattern of an amorphous sample depicting many intentionally overlapping disks blended into one image providing information about electron optical system.
In gases
 Gas electron diffraction (GED) can be used to determine
Gas electron diffraction (GED) can be used to determine geometry
Geometry (; ) is, with arithmetic, one of the oldest branches of mathematics. It is concerned with properties of space such as the distance, shape, size, and relative position of figures. A mathematician who works in the field of geometry is c ...
of molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and b ...
s dispersed in gases. A gas carrying the examined molecules is exposed to the electron beam, which is diffracted on the molecules. Since the diffracting molecules are randomly oriented, the resulting diffraction pattern consists of concentric rings. The experimentally acquired diffraction intensity is a summation of several components such as background, atomic intensity or molecular intensity.
The most valuable information about the molecular geometry is carried by the molecular intensity , which is specific to pairs of molecular atoms as described above in Theory. If this intensity is derived from an experimental diffractogram by subtracting other contributing intensities, it can be used to match and refine a structural model against the experimental data using an appropriate software.
As an alternative to GED, microwave spectroscopy can be used.
In scanning electron microscope
 In Scanning electron microscope the sample surface is mapped using a scanning electron beam. The diffraction image is formed using an electron backscatter diffraction (EBSD). A thin layer of the sample material is penetrated by the electrons, some of which are reflected due to the interactions inside the material and are diffracted on their journey out of the sample. As result of the inelastic scattering followed by the elastic one, typical features for an EBSD image are Kikuchi lines. Since the position of Kikuchi bands is highly sensitive to the crystal orientation, EBSD data acquired using the scanning beam can be used to determine the crystal orientation at particular location on the sample surface. The data are processed by an automated software allowing to routinely generate two-dimensional orientation maps across the sample surface. As the Kikuchi lines carry information about the interplanar angles and distances and, therefore, about the crystal structure, they can be also used for a phase identification or strain analysis.
In Scanning electron microscope the sample surface is mapped using a scanning electron beam. The diffraction image is formed using an electron backscatter diffraction (EBSD). A thin layer of the sample material is penetrated by the electrons, some of which are reflected due to the interactions inside the material and are diffracted on their journey out of the sample. As result of the inelastic scattering followed by the elastic one, typical features for an EBSD image are Kikuchi lines. Since the position of Kikuchi bands is highly sensitive to the crystal orientation, EBSD data acquired using the scanning beam can be used to determine the crystal orientation at particular location on the sample surface. The data are processed by an automated software allowing to routinely generate two-dimensional orientation maps across the sample surface. As the Kikuchi lines carry information about the interplanar angles and distances and, therefore, about the crystal structure, they can be also used for a phase identification or strain analysis.
See also
* Diffraction * Selected area diffraction *Convergent beam electron diffraction
Convergent beam electron diffraction (CBED) is a diffraction technique where a convergent or divergent beam (conical electron beam) of electrons is used to study materials.
History
This technique was first introduced in 1939 by Kossel and Möll ...
* Electron backscatter diffraction
* Gas electron diffraction
* Low-energy electron diffraction
* Precession Electron Diffraction
* Reflection high-energy electron diffraction
* Ronchigram
* Kikuchi line
* Electron crystallography
* Electron microscope
An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination. As the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, electron microscopes have a hi ...
* Transmission electron microscopy
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a ...
* Scanning electron microscopy
* CrysTBox
* Microcrystal electron diffraction
* Diffraction
* Crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pattern ...
* Stereographic projection
In mathematics, a stereographic projection is a perspective projection of the sphere, through a specific point on the sphere (the ''pole'' or ''center of projection''), onto a plane (the ''projection plane'') perpendicular to the diameter thro ...
* Zone axis
References
External links
Virtual lab on electron diffraction
*
Jmol
Jmol is computer software for molecular modelling chemical structures in 3-dimensions. Jmol returns a 3D representation of a molecule that may be used as a teaching tool, or for research e.g., in chemistry and biochemistry.
It is written in ...
-mediated image/diffractioanalysis of an unknown
* PTCLab-Program for calculation phase transformation crystallography with diffraction simulation, its free and open source python program https://code.google.com/p/transformation-crystallography-lab/
ronchigram.com
Web simulator for generating convergent beam diffraction of amorphous materials. {{Authority control Diffraction Electron Quantum mechanics