discovery of the neutron on:
[Wikipedia]
[Google]
[Amazon]
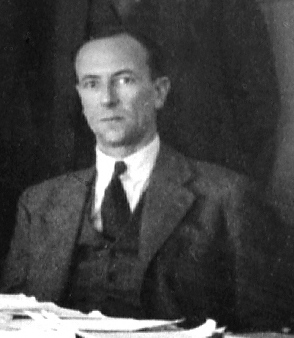 The discovery of the neutron and its properties was central to the extraordinary developments in
The discovery of the neutron and its properties was central to the extraordinary developments in
 In 1920 Rutherford gave a Bakerian lecture at the Royal Society entitled the "Nuclear Constitution of Atoms", a summary of recent experiments on atomic nuclei and conclusions as to the structure of atomic nuclei. By 1920, the existence of electrons within the atomic nucleus was widely assumed. It was assumed the nucleus consisted of hydrogen nuclei in number equal to the atomic mass. But since each hydrogen nucleus had charge +1, the nucleus required a smaller number of "internal electrons" each of charge −1 to give the nucleus its correct total charge. The mass of protons is about 1800 times greater than that of electrons, so the mass of the electrons is incidental in this computation. Such a model was consistent with the scattering of alpha particles from heavy nuclei, as well as the charge and mass of the many isotopes that had been identified. There were other motivations for the proton–electron model. As noted by Rutherford at the time, "We have strong reason for believing that the nuclei of atoms contain electrons as well as positively charged bodies...", namely, it was known that beta radiation was electrons emitted from the nucleus.
In that lecture, Rutherford conjectured the existence of new particles. The alpha particle was known to be very stable, and it was assumed to retain its identity within the nucleus. The alpha particle was presumed to consist of four protons and two closely bound electrons to give it +2 charge and mass 4. In a 1919 paper, Rutherford had reported the apparent discovery of a new doubly charged particle of mass 3, denoted the X++, interpreted to consist of three protons and a closely bound electron. This result suggested to Rutherford the likely existence of two new particles: one of two protons with a closely bound electron, and another of one proton and a closely bound electron. The X++ particle was later determined to have mass 4 and to be just a low-energy alpha particle. Nevertheless, Rutherford had conjectured the existence of the deuteron, a +1 charge particle of mass 2, and the neutron, a neutral particle of mass 1. The former is the nucleus of
In 1920 Rutherford gave a Bakerian lecture at the Royal Society entitled the "Nuclear Constitution of Atoms", a summary of recent experiments on atomic nuclei and conclusions as to the structure of atomic nuclei. By 1920, the existence of electrons within the atomic nucleus was widely assumed. It was assumed the nucleus consisted of hydrogen nuclei in number equal to the atomic mass. But since each hydrogen nucleus had charge +1, the nucleus required a smaller number of "internal electrons" each of charge −1 to give the nucleus its correct total charge. The mass of protons is about 1800 times greater than that of electrons, so the mass of the electrons is incidental in this computation. Such a model was consistent with the scattering of alpha particles from heavy nuclei, as well as the charge and mass of the many isotopes that had been identified. There were other motivations for the proton–electron model. As noted by Rutherford at the time, "We have strong reason for believing that the nuclei of atoms contain electrons as well as positively charged bodies...", namely, it was known that beta radiation was electrons emitted from the nucleus.
In that lecture, Rutherford conjectured the existence of new particles. The alpha particle was known to be very stable, and it was assumed to retain its identity within the nucleus. The alpha particle was presumed to consist of four protons and two closely bound electrons to give it +2 charge and mass 4. In a 1919 paper, Rutherford had reported the apparent discovery of a new doubly charged particle of mass 3, denoted the X++, interpreted to consist of three protons and a closely bound electron. This result suggested to Rutherford the likely existence of two new particles: one of two protons with a closely bound electron, and another of one proton and a closely bound electron. The X++ particle was later determined to have mass 4 and to be just a low-energy alpha particle. Nevertheless, Rutherford had conjectured the existence of the deuteron, a +1 charge particle of mass 2, and the neutron, a neutral particle of mass 1. The former is the nucleus of
 Two years later Irène Joliot-Curie and Frédéric Joliot in Paris showed that if this unknown radiation fell on
Two years later Irène Joliot-Curie and Frédéric Joliot in Paris showed that if this unknown radiation fell on
 Given the problems of the ''proton–electron model'',Friedlander, G.; Kennedy, J.W.; Miller, J.M. (1964) ''Nuclear and Radiochemistry'' (2nd edition), Wiley, pp. 22–23 and 38–39 it was quickly accepted that the atomic nucleus is composed of protons and neutrons, although the precise nature of the neutron was initially unclear. Within months after the discovery of the neutron,
Given the problems of the ''proton–electron model'',Friedlander, G.; Kennedy, J.W.; Miller, J.M. (1964) ''Nuclear and Radiochemistry'' (2nd edition), Wiley, pp. 22–23 and 38–39 it was quickly accepted that the atomic nucleus is composed of protons and neutrons, although the precise nature of the neutron was initially unclear. Within months after the discovery of the neutron,
 The question of whether the neutron was a composite particle of a proton and an electron persisted for a few years after its discovery. In 1932 Harrie Massey explored a model for a composite neutron to account for its great penetrating power through matter and its electrical neutrality, for example. The issue was a legacy of the prevailing view from the 1920s that the only elementary particles were the proton and electron.
The nature of the neutron was a primary topic of discussion at the 7th Solvay Conference held in October 1933, attended by Heisenberg,
The question of whether the neutron was a composite particle of a proton and an electron persisted for a few years after its discovery. In 1932 Harrie Massey explored a model for a composite neutron to account for its great penetrating power through matter and its electrical neutrality, for example. The issue was a legacy of the prevailing view from the 1920s that the only elementary particles were the proton and electron.
The nature of the neutron was a primary topic of discussion at the 7th Solvay Conference held in October 1933, attended by Heisenberg,
 The discovery of the neutron immediately gave scientists a new tool for probing the properties of atomic nuclei. Alpha particles had been used over the previous decades in scattering experiments, but such particles, which are helium nuclei, have +2 charge. This charge makes it difficult for alpha particles to overcome the Coulomb repulsive force and interact directly with the nuclei of atoms. Since neutrons have no electric charge, they do not have to overcome this force to interact with nuclei. Almost coincident with their discovery, neutrons were used by Norman Feather, Chadwick's colleague and protege, in scattering experiments with nitrogen. Feather was able to show that neutrons interacting with nitrogen nuclei scattered to protons or induced nitrogen to disintegrate to form
The discovery of the neutron immediately gave scientists a new tool for probing the properties of atomic nuclei. Alpha particles had been used over the previous decades in scattering experiments, but such particles, which are helium nuclei, have +2 charge. This charge makes it difficult for alpha particles to overcome the Coulomb repulsive force and interact directly with the nuclei of atoms. Since neutrons have no electric charge, they do not have to overcome this force to interact with nuclei. Almost coincident with their discovery, neutrons were used by Norman Feather, Chadwick's colleague and protege, in scattering experiments with nitrogen. Feather was able to show that neutrons interacting with nitrogen nuclei scattered to protons or induced nitrogen to disintegrate to form 
 In
In
 The discovery of nuclear fission at the end of 1938 marked a shift in the centers of nuclear research from
The discovery of nuclear fission at the end of 1938 marked a shift in the centers of nuclear research from
Ernest Rutherford summarizes the state of nuclear physics in 1935.
(7 min., Nobelprize.org)
Hans Bethe discusses Chadwick and Goldhaber's work on deuteron disintegration.
(2 min., Web of Stories)
Annotated bibliography for neutrons from the Alsos Digital Library for Nuclear Issues
* Abraham Pais, ''Inward Bound'', Oxford: Oxford University Press, 1986. . *
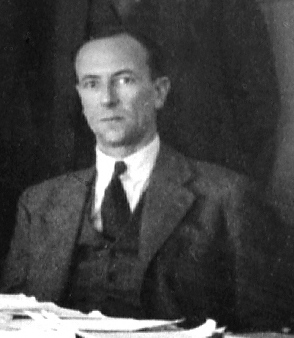 The discovery of the neutron and its properties was central to the extraordinary developments in
The discovery of the neutron and its properties was central to the extraordinary developments in atomic physics
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. Atomic physics typically refers to the study of atomic structure and the interaction between atoms. It is primarily concerned wit ...
in the first half of the 20th century. Early in the century, Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson (30 August 1871 – 19 October 1937) was a New Zealand physicist who was a pioneering researcher in both Atomic physics, atomic and nuclear physics. He has been described as "the father of nu ...
developed a crude model
A model is an informative representation of an object, person, or system. The term originally denoted the plans of a building in late 16th-century English, and derived via French and Italian ultimately from Latin , .
Models can be divided in ...
of the atom, based on the gold foil experiment of Hans Geiger
Johannes Wilhelm Geiger ( , ; ; 30 September 1882 – 24 September 1945) was a German nuclear physicist. He is known as the inventor of the Geiger counter, a device used to detect ionizing radiation, and for carrying out the Rutherford scatt ...
and Ernest Marsden. In this model, atoms had their mass
Mass is an Intrinsic and extrinsic properties, intrinsic property of a physical body, body. It was traditionally believed to be related to the physical quantity, quantity of matter in a body, until the discovery of the atom and particle physi ...
and positive electric charge concentrated in a very small nucleus. By 1920, isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s of chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
s had been discovered, the atomic mass
Atomic mass ( or ) is the mass of a single atom. The atomic mass mostly comes from the combined mass of the protons and neutrons in the nucleus, with minor contributions from the electrons and nuclear binding energy. The atomic mass of atoms, ...
es had been determined to be (approximately) integer multiples of the mass of the hydrogen atom, and the atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
had been identified as the charge on the nucleus.Byrne, J. ''Neutrons, Nuclei, and Matter'', Dover Publications, Mineola, New York, 2011, Throughout the 1920s, the nucleus was viewed as composed of combinations of proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s and electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s, the two elementary particle
In particle physics, an elementary particle or fundamental particle is a subatomic particle that is not composed of other particles. The Standard Model presently recognizes seventeen distinct particles—twelve fermions and five bosons. As a c ...
s known at the time, but that model presented several experimental and theoretical contradictions.
The essential nature of the atomic nucleus was established with the discovery of the neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
by James Chadwick
Sir James Chadwick (20 October 1891 – 24 July 1974) was an English nuclear physicist who received the Nobel Prize in Physics in 1935 for his discovery of the neutron. In 1941, he wrote the final draft of the MAUD Report, which inspired t ...
in 1932 and the determination that it was a new elementary particle, distinct from the proton.
The uncharged neutron was immediately exploited as a new means to probe nuclear structure, leading to such discoveries as the creation of new radioactive elements by neutron irradiation (1934) and the fission of uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
atoms by neutrons (1938). The discovery of fission led to the creation of both nuclear power
Nuclear power is the use of nuclear reactions to produce electricity. Nuclear power can be obtained from nuclear fission, nuclear decay and nuclear fusion reactions. Presently, the vast majority of electricity from nuclear power is produced by ...
and nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission or atomic bomb) or a combination of fission and fusion reactions (thermonuclear weapon), producing a nuclear exp ...
s by the end of World War II. Both the proton and the neutron were presumed to be elementary particles until the 1960s, when they were determined to be composite particles built from quark
A quark () is a type of elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nucleus, atomic nuclei ...
s.
Discovery of radioactivity
At the start of the 20th century, the vigorous debate as to the existence of atoms had not yet been resolved. Philosophers such asErnst Mach
Ernst Waldfried Josef Wenzel Mach ( ; ; 18 February 1838 – 19 February 1916) was an Austrian physicist and philosopher, who contributed to the understanding of the physics of shock waves. The ratio of the speed of a flow or object to that of ...
and Wilhelm Ostwald denied that atoms were real, viewing them as a convenient mathematical construct, while scientists such as Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld (; 5 December 1868 – 26 April 1951) was a German Theoretical physics, theoretical physicist who pioneered developments in Atomic physics, atomic and Quantum mechanics, quantum physics, and also educated and ...
and Ludwig Boltzmann
Ludwig Eduard Boltzmann ( ; ; 20 February 1844 – 5 September 1906) was an Austrian mathematician and Theoretical physics, theoretical physicist. His greatest achievements were the development of statistical mechanics and the statistical ex ...
saw that physical theories required the existence of atoms.
Radioactivity
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
was discovered in 1896 by the French scientist Henri Becquerel
Antoine Henri Becquerel ( ; ; 15 December 1852 – 25 August 1908) was a French nuclear physicist who shared the 1903 Nobel Prize in Physics with Marie and Pierre Curie for his discovery of radioactivity.
Biography
Family and education
Becq ...
, while working with phosphorescent materials. In 1898, Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson (30 August 1871 – 19 October 1937) was a New Zealand physicist who was a pioneering researcher in both Atomic physics, atomic and nuclear physics. He has been described as "the father of nu ...
at Cavendish Laboratory
The Cavendish Laboratory is the Department of Physics at the University of Cambridge, and is part of the School of Physical Sciences. The laboratory was opened in 1874 on the New Museums Site as a laboratory for experimental physics and is named ...
distinguished two types of radioactivity, alpha rays and beta rays, which differed in their ability to penetrate, or travel into, ordinary objects or gases. Two years later, Paul Villard discovered gamma
Gamma (; uppercase , lowercase ; ) is the third letter of the Greek alphabet. In the system of Greek numerals it has a value of 3. In Ancient Greek, the letter gamma represented a voiced velar stop . In Modern Greek, this letter normally repr ...
rays, which possessed even more penetrating power. These radiations were soon identified with known particles: beta rays were shown to be electrons by Walter Kaufmann in 1902; alpha rays were shown to be helium ions by Rutherford and Thomas Royds in 1907; and gamma rays were shown to be electromagnetic radiation, that is, a form of light
Light, visible light, or visible radiation is electromagnetic radiation that can be visual perception, perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400– ...
, by Rutherford and Edward Andrade in 1914. These radiations had also been identified as emanating from atoms, hence they provided clues to processes occurring within atoms. Conversely, the radiations were also recognized as tools that could be exploited in scattering experiments to probe the interior of atoms.
Gold foil experiment and the discovery of the atomic nucleus
At theUniversity of Manchester
The University of Manchester is a public university, public research university in Manchester, England. The main campus is south of Manchester city centre, Manchester City Centre on Wilmslow Road, Oxford Road. The University of Manchester is c ...
between 1908 and 1913, Rutherford directed Hans Geiger
Johannes Wilhelm Geiger ( , ; ; 30 September 1882 – 24 September 1945) was a German nuclear physicist. He is known as the inventor of the Geiger counter, a device used to detect ionizing radiation, and for carrying out the Rutherford scatt ...
and Ernest Marsden in a series of experiments to determine what happens when alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s scatter from metal foil. Now called the Rutherford gold foil experiment, or the Geiger–Marsden experiment, these measurements made the extraordinary discovery that although most alpha particles passing through a thin gold foil experienced little deflection, a few scattered to a high angle. The scattering indicated that some of the alpha particles ricocheted back from a small, but dense, component inside the atoms. Based on these measurements, by 1911 it was apparent to Rutherford that the atom consisted of a small, massive nucleus with positive charge surrounded by a much larger cloud of negatively charged electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s. The concentrated atomic mass was required to provide the observed deflection of the alpha particles, and Rutherford developed a mathematical model that accounted for the scattering.
While the Rutherford model was largely ignored at the time, when Niels Bohr
Niels Henrik David Bohr (, ; ; 7 October 1885 – 18 November 1962) was a Danish theoretical physicist who made foundational contributions to understanding atomic structure and old quantum theory, quantum theory, for which he received the No ...
joined Rutherford's group he developed the Bohr model
In atomic physics, the Bohr model or Rutherford–Bohr model was a model of the atom that incorporated some early quantum concepts. Developed from 1911 to 1918 by Niels Bohr and building on Ernest Rutherford's nuclear Rutherford model, model, i ...
for electrons orbiting the nucleus in 1913 and this eventually led to an atomic model based on quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
by the mid-1920s.
Discovery of isotopes
Concurrent with the work of Rutherford, Geiger, and Marsden, the radiochemist Frederick Soddy at theUniversity of Glasgow
The University of Glasgow (abbreviated as ''Glas.'' in Post-nominal letters, post-nominals; ) is a Public university, public research university in Glasgow, Scotland. Founded by papal bull in , it is the List of oldest universities in continuous ...
was studying chemistry related problems on radioactive materials. Soddy had worked with Rutherford on radioactivity at McGill University
McGill University (French: Université McGill) is an English-language public research university in Montreal, Quebec, Canada. Founded in 1821 by royal charter,Frost, Stanley Brice. ''McGill University, Vol. I. For the Advancement of Learning, ...
. By 1910, about 40 different radioactive elements, referred to as ''radioelements'', had been identified between uranium and lead, although the periodic table only allowed for 11 elements. Soddy and Kazimierz Fajans independently found in 1913 that an element undergoing alpha decay will produce an element two places to the left in the periodic system and an element undergoing beta decay will produce an element one place to the right in the periodic system. Also, those radioelements that reside in the same places in the periodic system are chemically identical. Soddy called these chemically identical elements isotopes
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), but ...
. For his study of radioactivity and the discovery of isotopes, Soddy was awarded the 1921 Nobel Prize in Chemistry.
Building from work by J. J. Thomson on the deflection of positively charged atoms by electric and magnetic fields, Francis Aston built the first mass spectrograph at the Cavendish Laboratory in 1919. He was able then to separate the two isotopes of neon
Neon is a chemical element; it has symbol Ne and atomic number 10. It is the second noble gas in the periodic table. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with approximately two-thirds the density of ...
, and . Aston discovered the whole number rule, that the masses of all the particles have whole number relationships to oxygen-16, which he took to have a mass of exactly 16. (Today the whole-number rule is expressed in multiples of an atomic mass unit
The dalton or unified atomic mass unit (symbols: Da or u, respectively) is a unit of mass defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. It is a non-SI unit accepted ...
(amu) relative to carbon-12
Carbon-12 (12C) is the most abundant of the two stable isotopes of carbon ( carbon-13 being the other), amounting to 98.93% of element carbon on Earth; its abundance is due to the triple-alpha process by which it is created in stars. Carbon-1 ...
.). Significantly, the one exception to this rule was hydrogen itself, which had a mass value of 1.008. The excess mass was small, but well outside the limits of experimental uncertainty.
Since Einstein's mass-energy equivalence had been known since 1905, Aston and others quickly realized that the mass discrepancy is due to the binding energy of atoms. When the contents of a number of hydrogen atoms are bound into a single atom, the single atom's energy must be less than the sum of the energies of the separate hydrogen atoms, and therefore the single atom's mass is less than the sum of the hydrogen atom masses. Aston's work on isotopes won him the 1922 Nobel Prize in Chemistry for the discovery of isotopes in a large number of non-radioactive elements, and for his enunciation of the whole number rule. Noting Aston's recent discovery of nuclear binding energy, in 1920 Arthur Eddington suggested that stars may obtain their energy by fusing hydrogen (protons) into helium and that the heavier elements may form in stars.
Atomic number and Moseley's law
Rutherford and others had noted the disparity between the mass of an atom, computed in atomic mass units, and the approximate charge required on the nucleus for the Rutherford model to work. The required charge of the atomic nucleus was usually about half its atomic mass. Antonius van den Broek boldly hypothesized that the required charge, denoted by ''Z'', was not half of the atomic weight for elements, but instead was exactly equal to the element's ordinal position in theperiodic table
The periodic table, also known as the periodic table of the elements, is an ordered arrangement of the chemical elements into rows (" periods") and columns (" groups"). It is an icon of chemistry and is widely used in physics and other s ...
. At that time, the positions of the elements in the periodic table were not known to have any physical significance. If the elements were ordered based on increasing atomic mass, however, periodicity in chemical properties was exhibited. Exceptions to this periodicity were apparent, however, such as cobalt and nickel.
At the University of Manchester
The University of Manchester is a public university, public research university in Manchester, England. The main campus is south of Manchester city centre, Manchester City Centre on Wilmslow Road, Oxford Road. The University of Manchester is c ...
in 1913 Henry Moseley discussed the new Bohr model
In atomic physics, the Bohr model or Rutherford–Bohr model was a model of the atom that incorporated some early quantum concepts. Developed from 1911 to 1918 by Niels Bohr and building on Ernest Rutherford's nuclear Rutherford model, model, i ...
of the atom with the visiting Bohr. The model accounted for the electromagnetic emission spectrum from the hydrogen atom, and Moseley and Bohr wondered if the electromagnetic emission spectra of heavier elements such as cobalt and nickel would follow their ordering by weight, or by their position in the periodic table. In 1913–1914 Moseley tested the question experimentally by using X-ray diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. ...
techniques. He found that the most intense short-wavelength line in the X-ray spectrum of a particular element, known as the K-alpha line, was related to the element's position in the periodic table, that is, its atomic number, ''Z''. Indeed, Moseley introduced this nomenclature. Moseley found that the frequencies of the radiation were related in a simple way to the atomic number of the elements for a large number of elements.
Within a year it was noted that the equation for the relation, now called Moseley's law, could be explained in terms of the 1913 Bohr model, with reasonable extra assumptions about atomic structure in other elements. Moseley's result, by Bohr's later account, not only established atomic number as a measurable experimental quantity, but gave it a physical meaning as the positive charge on the atomic nucleus. The elements could be ordered in the periodic system in order of atomic number, rather than atomic weight. The result tied together the organization of the periodic table, the Bohr model for the atom, and Rutherford's model for alpha scattering from nuclei. It was cited by Rutherford, Bohr, and others as a critical advance in understanding the nature of the atomic nucleus.
Further research in atomic physics was interrupted by the outbreak of World War I
World War I or the First World War (28 July 1914 – 11 November 1918), also known as the Great War, was a World war, global conflict between two coalitions: the Allies of World War I, Allies (or Entente) and the Central Powers. Fighting to ...
. Moseley was killed in 1915 at the Battle of Gallipoli, while Rutherford's student James Chadwick
Sir James Chadwick (20 October 1891 – 24 July 1974) was an English nuclear physicist who received the Nobel Prize in Physics in 1935 for his discovery of the neutron. In 1941, he wrote the final draft of the MAUD Report, which inspired t ...
was interned in Germany for the duration of the war, 1914–1918. In Berlin, Lise Meitner
Elise Lise Meitner ( ; ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish nuclear physicist who was instrumental in the discovery of nuclear fission.
After completing her doctoral research in 1906, Meitner became the second woman ...
's and Otto Hahn
Otto Hahn (; 8 March 1879 – 28 July 1968) was a German chemist who was a pioneer in the field of radiochemistry. He is referred to as the father of nuclear chemistry and discoverer of nuclear fission, the science behind nuclear reactors and ...
's research work on determining the radioactive decay chains of radium and uranium by precise chemical separation was interrupted. Meitner spent much of the war working as a radiologist
Radiology ( ) is the medical specialty that uses medical imaging to diagnose diseases and guide treatment within the bodies of humans and other animals. It began with radiography (which is why its name has a root referring to radiation), but tod ...
and medical X-ray
An X-ray (also known in many languages as Röntgen radiation) is a form of high-energy electromagnetic radiation with a wavelength shorter than those of ultraviolet rays and longer than those of gamma rays. Roughly, X-rays have a wavelength ran ...
technician near the Austrian front, while Hahn, a chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a graduated scientist trained in the study of chemistry, or an officially enrolled student in the field. Chemists study the composition of ...
, worked on research in poison gas warfare.
Rutherford nucleus
 In 1920 Rutherford gave a Bakerian lecture at the Royal Society entitled the "Nuclear Constitution of Atoms", a summary of recent experiments on atomic nuclei and conclusions as to the structure of atomic nuclei. By 1920, the existence of electrons within the atomic nucleus was widely assumed. It was assumed the nucleus consisted of hydrogen nuclei in number equal to the atomic mass. But since each hydrogen nucleus had charge +1, the nucleus required a smaller number of "internal electrons" each of charge −1 to give the nucleus its correct total charge. The mass of protons is about 1800 times greater than that of electrons, so the mass of the electrons is incidental in this computation. Such a model was consistent with the scattering of alpha particles from heavy nuclei, as well as the charge and mass of the many isotopes that had been identified. There were other motivations for the proton–electron model. As noted by Rutherford at the time, "We have strong reason for believing that the nuclei of atoms contain electrons as well as positively charged bodies...", namely, it was known that beta radiation was electrons emitted from the nucleus.
In that lecture, Rutherford conjectured the existence of new particles. The alpha particle was known to be very stable, and it was assumed to retain its identity within the nucleus. The alpha particle was presumed to consist of four protons and two closely bound electrons to give it +2 charge and mass 4. In a 1919 paper, Rutherford had reported the apparent discovery of a new doubly charged particle of mass 3, denoted the X++, interpreted to consist of three protons and a closely bound electron. This result suggested to Rutherford the likely existence of two new particles: one of two protons with a closely bound electron, and another of one proton and a closely bound electron. The X++ particle was later determined to have mass 4 and to be just a low-energy alpha particle. Nevertheless, Rutherford had conjectured the existence of the deuteron, a +1 charge particle of mass 2, and the neutron, a neutral particle of mass 1. The former is the nucleus of
In 1920 Rutherford gave a Bakerian lecture at the Royal Society entitled the "Nuclear Constitution of Atoms", a summary of recent experiments on atomic nuclei and conclusions as to the structure of atomic nuclei. By 1920, the existence of electrons within the atomic nucleus was widely assumed. It was assumed the nucleus consisted of hydrogen nuclei in number equal to the atomic mass. But since each hydrogen nucleus had charge +1, the nucleus required a smaller number of "internal electrons" each of charge −1 to give the nucleus its correct total charge. The mass of protons is about 1800 times greater than that of electrons, so the mass of the electrons is incidental in this computation. Such a model was consistent with the scattering of alpha particles from heavy nuclei, as well as the charge and mass of the many isotopes that had been identified. There were other motivations for the proton–electron model. As noted by Rutherford at the time, "We have strong reason for believing that the nuclei of atoms contain electrons as well as positively charged bodies...", namely, it was known that beta radiation was electrons emitted from the nucleus.
In that lecture, Rutherford conjectured the existence of new particles. The alpha particle was known to be very stable, and it was assumed to retain its identity within the nucleus. The alpha particle was presumed to consist of four protons and two closely bound electrons to give it +2 charge and mass 4. In a 1919 paper, Rutherford had reported the apparent discovery of a new doubly charged particle of mass 3, denoted the X++, interpreted to consist of three protons and a closely bound electron. This result suggested to Rutherford the likely existence of two new particles: one of two protons with a closely bound electron, and another of one proton and a closely bound electron. The X++ particle was later determined to have mass 4 and to be just a low-energy alpha particle. Nevertheless, Rutherford had conjectured the existence of the deuteron, a +1 charge particle of mass 2, and the neutron, a neutral particle of mass 1. The former is the nucleus of deuterium
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium nucleus (deuteron) contains one proton and one neutron, whereas the far more c ...
, discovered in 1931 by Harold Urey. The mass of the hypothetical neutral particle would be little different from that of the proton. Rutherford determined that such a zero-charge particle would be difficult to detect by available techniques.
About the time of Rutherford's lecture, other publications appeared with similar suggestions of a proton–electron composite in the nucleus, and in 1921 William Harkins, an American chemist, named the uncharged particle the ''neutron''. About that same time the word ''proton'' was adopted for the hydrogen nucleus. Neutron was apparently constructed from the Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
root for ''neutral'' and the Greek ending ''-on'' (by imitation of ''electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
'' and ''proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
''). References to the word ''neutron'' in connection with the atom can be found in the literature as early as 1899, however.
Rutherford and Chadwick immediately began an experimental program at the Cavendish Laboratory
The Cavendish Laboratory is the Department of Physics at the University of Cambridge, and is part of the School of Physical Sciences. The laboratory was opened in 1874 on the New Museums Site as a laboratory for experimental physics and is named ...
in Cambridge
Cambridge ( ) is a List of cities in the United Kingdom, city and non-metropolitan district in the county of Cambridgeshire, England. It is the county town of Cambridgeshire and is located on the River Cam, north of London. As of the 2021 Unit ...
to search for the neutron. The experiments continued throughout the 1920s without success.
Rutherford's conjecture and the hypothetical "neutron" were not widely accepted. In his 1931 monograph on the ''Constitution of Atomic Nuclei and Radioactivity'', George Gamow, then at the Institute for Theoretical Physics in Copenhagen, did not mention the neutron.George Gamow "Constitution of Atomic Nuclei and Radioactivity".
(The International Series of Monographs on Physics.) Pp.viii + 114.(Oxford: Clarendon Press; London: Oxford University Press, 1931.) At the time of their 1932 measurements in Paris that would lead to the discovery of the neutron, Irène Joliot-Curie and Frédéric Joliot were unaware of the conjecture.
Problems of the nuclear electrons hypothesis
Throughout the 1920s, physicists assumed that the atomic nucleus was composed of protons and "nuclear electrons". Under this hypothesis, the nitrogen-14 (14N) nucleus would be composed of 14 protons and 7 electrons, so that it would have a net charge of +7elementary charge
The elementary charge, usually denoted by , is a fundamental physical constant, defined as the electric charge carried by a single proton (+1 ''e'') or, equivalently, the magnitude of the negative electric charge carried by a single electron, ...
units and a mass of 14 atomic mass units. This nucleus would also be orbited by another 7 electrons, termed "external electrons" by Rutherford, to complete the 14N atom. However problems with the hypothesis soon became apparent.
Ralph Kronig pointed out in 1926 that the observed hyperfine structure of atomic spectra was inconsistent with the proton–electron hypothesis. This structure is caused by the influence of the nucleus on the dynamics of orbiting electrons. The magnetic moments of supposed "nuclear electrons" should produce hyperfine spectral line splittings similar to the Zeeman effect
The Zeeman effect () is the splitting of a spectral line into several components in the presence of a static magnetic field. It is caused by the interaction of the magnetic field with the magnetic moment of the atomic electron associated with ...
, but no such effects were observed. It seemed that the magnetic moment of the electron vanished when it was within the nucleus.
While on a visit to Utrecht University
Utrecht University (UU; , formerly ''Rijksuniversiteit Utrecht'') is a public university, public research university in Utrecht, Netherlands. Established , it is one of the oldest universities in the Netherlands. In 2023, it had an enrollment of ...
in 1928, Kronig learned of a surprising aspect of the rotational spectrum of N2+. The precision measurement made by Leonard Ornstein, the director of Utrecht's Physical Laboratory, showed that the spin of nitrogen nucleus must be equal to one. However, if the nitrogen-14 (14N) nucleus was composed of 14 protons and 7 electrons, an odd number of spin-1/2 particles, then the resultant nuclear spin should be half-integer. Kronig therefore suggested that perhaps "protons and electrons do not retain their identity to the extent they do outside the nucleus".
Observations of the rotational energy levels of diatomic molecules using Raman spectroscopy
Raman spectroscopy () (named after physicist C. V. Raman) is a Spectroscopy, spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Ra ...
by Franco Rasetti in 1929 were inconsistent with the statistics expected from the proton–electron hypothesis. Rasetti obtained band spectra for H2 and N2 molecules. While the lines for both diatomic molecules showed alternation in intensity between light and dark, the pattern of alternation for H2 is opposite to that of the N2. After carefully analyzing these experimental results, German physicists Walter Heitler and Gerhard Herzberg showed that the hydrogen nuclei obey Fermi statistics and the nitrogen nuclei obey Bose statistics. However, a then unpublished result of Eugene Wigner
Eugene Paul Wigner (, ; November 17, 1902 – January 1, 1995) was a Hungarian-American theoretical physicist who also contributed to mathematical physics. He received the Nobel Prize in Physics in 1963 "for his contributions to the theory of th ...
showed that a composite system with an odd number of spin-1/2 particles must obey Fermi statistics; a system with an even number of spin-1/2 particle obeys Bose statistics. If the nitrogen nucleus had 21 particles, it should obey Fermi statistics, contrary to fact. Thus, Heitler and Herzberg concluded: "the electron in the nucleus ... loses its ability to determine the statistics of the nucleus."
The Klein paradox, discovered by Oskar Klein
Oskar Benjamin Klein (; 15 September 1894 – 5 February 1977) was a Swedish theoretical physics, theoretical physicist.
Oskar Klein is known for his work on Kaluza–Klein theory, which is partially named after him.
Biography
Klein was born ...
in 1928, presented further quantum mechanical objections to the notion of an electron confined within a nucleus. Derived from the Dirac equation
In particle physics, the Dirac equation is a relativistic wave equation derived by British physicist Paul Dirac in 1928. In its free form, or including electromagnetic interactions, it describes all spin-1/2 massive particles, called "Dirac ...
, this clear and precise paradox suggested that an electron approaching a high potential barrier has a high probability of passing through the barrier by a pair creation process. Apparently, an electron could not be confined within a nucleus by any potential well. The meaning of this paradox was intensely debated at the time.
By about 1930 it was generally recognized that it was difficult to reconcile the proton–electron model for nuclei with the Heisenberg uncertainty relation of quantum mechanics. This relation, , implies that an electron confined to a region the size of an atomic nucleus typically has a kinetic energy of about 40 MeV, which is larger than the observed energy of beta particles emitted from the nucleus. Such energy is also much larger than the binding energy of nucleons,
which Aston and others had shown to be less than 9 MeV per nucleon.
In 1927, Charles Ellis and W. Wooster at the Cavendish Laboratory measured the energies of β-decay electrons. They found that the distribution of energies from any particular radioactive nuclei was broad and continuous, a result that contrasted notably with the distinct energy values observed in alpha and gamma decay. Further, the continuous energy distribution seemed to indicate that energy was not conserved by this "nuclear electrons" process. Indeed, in 1929 Bohr proposed to modify the law of energy conservation to account for the continuous energy distribution. The proposal earned the support of Werner Heisenberg. Such considerations were apparently reasonable, inasmuch as the laws of quantum mechanics had so recently overturned the laws of classical mechanics.
While all these considerations did not "prove" an electron could not exist in the nucleus, they were confusing and challenging for physicist
A physicist is a scientist who specializes in the field of physics, which encompasses the interactions of matter and energy at all length and time scales in the physical universe. Physicists generally are interested in the root or ultimate cau ...
s to interpret. Many theories were invented to explain how the above arguments could be wrong. In his 1931 monograph, Gamow summarized all these contradictions, marking the statements regarding electrons in the nucleus with warning symbols.
Discovery of the neutron
In 1930, Walther Bothe and his collaborator Herbert Becker inGiessen
Giessen, spelled in German (), is a town in the Germany, German States of Germany, state () of Hesse, capital of both the Giessen (district), district of Giessen and the Giessen (region), administrative region of Giessen. The population is appro ...
, Germany found that if the energetic alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay but may also be produce ...
s emitted from polonium fell on certain light elements, specifically beryllium
Beryllium is a chemical element; it has Symbol (chemistry), symbol Be and atomic number 4. It is a steel-gray, hard, strong, lightweight and brittle alkaline earth metal. It is a divalent element that occurs naturally only in combination with ...
(), boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
(), or lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
(), an unusually penetrating radiation was produced. Beryllium produced the most intense radiation. Polonium is highly radioactive, producing energetic alpha radiation, and it was commonly used for scattering experiments at the time. Alpha radiation can be influenced by an electric field, because it is composed of charged particles. The observed penetrating radiation was not influenced by an electric field, however, so it was thought to be gamma radiation
A gamma ray, also known as gamma radiation (symbol ), is a penetrating form of electromagnetic radiation arising from high energy interactions like the radioactive decay of atomic nuclei or astronomical events like solar flares. It consists o ...
. The radiation was more penetrating than any gamma rays known, and the details of experimental results were difficult to interpret.
 Two years later Irène Joliot-Curie and Frédéric Joliot in Paris showed that if this unknown radiation fell on
Two years later Irène Joliot-Curie and Frédéric Joliot in Paris showed that if this unknown radiation fell on paraffin wax
Paraffin wax (or petroleum wax) is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and melting poi ...
, or any other hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
-containing compound, it ejected protons of very high energy (5 MeV). This observation was not in itself inconsistent with the assumed gamma ray nature of the new radiation, but that interpretation ( Compton scattering) had a logical problem. From energy and momentum considerations, a gamma ray would have to have impossibly high energy (50 MeV) to scatter a massive proton. In Rome, the young physicist Ettore Majorana declared that the manner in which the new radiation interacted with protons required a neutral particle as heavy as a proton, but declined to publish his result despite the encouragement of Enrico Fermi.
On hearing of the Paris results, Rutherford and James Chadwick
Sir James Chadwick (20 October 1891 – 24 July 1974) was an English nuclear physicist who received the Nobel Prize in Physics in 1935 for his discovery of the neutron. In 1941, he wrote the final draft of the MAUD Report, which inspired t ...
at the Cavendish Laboratory also did not believe the gamma ray hypothesis since it failed to conserve energy. Assisted by Norman Feather, Chadwick quickly performed a series of experiments showing that the gamma ray hypothesis was untenable. The previous year, Chadwick, J.E.R. Constable, and E.C. Pollard had already conducted experiments on disintegrating light elements using alpha radiation from polonium. They had also developed more accurate and efficient methods for detecting, counting, and recording the ejected protons. Chadwick repeated the creation of the radiation using beryllium to absorb the alpha particles: Following the Paris experiment, he aimed the radiation at paraffin wax, a hydrocarbon high in hydrogen content, hence offering a target dense with protons. As in the Paris experiment, the radiation energetically scattered some of the protons. Chadwick measured the range of these protons, and also measured how the new radiation impacted the atoms of various gases. Measurements of the recoil energy showed that the mass of the radiation particles must be similar to the mass of the proton: the new radiation could not consist of gamma rays. Uncharged particles with about the same mass as the proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
matched the properties Rutherford described in 1920 and which had later been called neutrons.
Chadwick won the Nobel Prize in Physics
The Nobel Prize in Physics () is an annual award given by the Royal Swedish Academy of Sciences for those who have made the most outstanding contributions to mankind in the field of physics. It is one of the five Nobel Prizes established by the ...
in 1935 for this discovery.
The year 1932 was later referred to as the " annus mirabilis" for nuclear physics in the Cavendish Laboratory, with discoveries of the neutron, artificial nuclear disintegration by the Cockcroft–Walton particle accelerator, and the positron
The positron or antielectron is the particle with an electric charge of +1''elementary charge, e'', a Spin (physics), spin of 1/2 (the same as the electron), and the same Electron rest mass, mass as an electron. It is the antiparticle (antimatt ...
.
Proton–neutron model of the nucleus
 Given the problems of the ''proton–electron model'',Friedlander, G.; Kennedy, J.W.; Miller, J.M. (1964) ''Nuclear and Radiochemistry'' (2nd edition), Wiley, pp. 22–23 and 38–39 it was quickly accepted that the atomic nucleus is composed of protons and neutrons, although the precise nature of the neutron was initially unclear. Within months after the discovery of the neutron,
Given the problems of the ''proton–electron model'',Friedlander, G.; Kennedy, J.W.; Miller, J.M. (1964) ''Nuclear and Radiochemistry'' (2nd edition), Wiley, pp. 22–23 and 38–39 it was quickly accepted that the atomic nucleus is composed of protons and neutrons, although the precise nature of the neutron was initially unclear. Within months after the discovery of the neutron, Werner Heisenberg
Werner Karl Heisenberg (; ; 5 December 1901 – 1 February 1976) was a German theoretical physicist, one of the main pioneers of the theory of quantum mechanics and a principal scientist in the German nuclear program during World War II.
He pub ...
and Dmitri Ivanenko had proposed proton–neutron models for the nucleus. Heisenberg's landmark papers approached the description of protons and neutrons in the nucleus through quantum mechanics. While Heisenberg's theory for protons and neutrons in the nucleus was a "major step toward understanding the nucleus as a quantum mechanical system", he still assumed the presence of nuclear electrons. In particular, Heisenberg assumed the neutron was a proton–electron composite, for which there is no quantum mechanical explanation. Heisenberg had no explanation for how lightweight electrons could be bound within the nucleus. Heisenberg introduced the first theory of nuclear exchange forces that bind the nucleons. He considered protons and neutrons to be different quantum states of the same particle, i.e., nucleons distinguished by the value of their nuclear isospin
In nuclear physics and particle physics, isospin (''I'') is a quantum number related to the up- and down quark content of the particle.
Isospin is also known as isobaric spin or isotopic spin.
Isospin symmetry is a subset of the flavour symmetr ...
quantum numbers.
The proton–neutron model explained the puzzle of dinitrogen. When 14N was proposed to consist of 3 pairs each of protons and neutrons, with an additional unpaired neutron and proton each contributing a spin of ħ in the same direction for a total spin of 1 ħ, the model became viable. Soon, neutrons were used to naturally explain spin differences in many different nuclides in the same way.
If the proton–neutron model for the nucleus resolved many issues, it highlighted the problem of explaining the origins of beta radiation. No existing theory could account for how electrons, or positrons, could emanate from the nucleus. In 1934, Enrico Fermi published his classic paper describing the process of beta decay, in which the neutron decays to a proton by ''creating'' an electron and a (as yet undiscovered) neutrino
A neutrino ( ; denoted by the Greek letter ) is an elementary particle that interacts via the weak interaction and gravity. The neutrino is so named because it is electrically neutral and because its rest mass is so small ('' -ino'') that i ...
. The paper employed the analogy that photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s, or electromagnetic radiation, were similarly created and destroyed in atomic processes. Ivanenko had suggested a similar analogy in 1932. Fermi's theory requires the neutron to be a spin- particle. The theory preserved the principle of conservation of energy, which had been thrown into question by the continuous energy distribution of beta particles. The basic theory for beta decay proposed by Fermi was the first to show how particles could be created and destroyed. It established a general, basic theory for the interaction of particles by weak or strong forces. While this influential paper has stood the test of time, the ideas within it were so new that when it was first submitted to the journal ''Nature'' in 1933 it was rejected as being too speculative.
Nature of the neutron
 The question of whether the neutron was a composite particle of a proton and an electron persisted for a few years after its discovery. In 1932 Harrie Massey explored a model for a composite neutron to account for its great penetrating power through matter and its electrical neutrality, for example. The issue was a legacy of the prevailing view from the 1920s that the only elementary particles were the proton and electron.
The nature of the neutron was a primary topic of discussion at the 7th Solvay Conference held in October 1933, attended by Heisenberg,
The question of whether the neutron was a composite particle of a proton and an electron persisted for a few years after its discovery. In 1932 Harrie Massey explored a model for a composite neutron to account for its great penetrating power through matter and its electrical neutrality, for example. The issue was a legacy of the prevailing view from the 1920s that the only elementary particles were the proton and electron.
The nature of the neutron was a primary topic of discussion at the 7th Solvay Conference held in October 1933, attended by Heisenberg, Niels Bohr
Niels Henrik David Bohr (, ; ; 7 October 1885 – 18 November 1962) was a Danish theoretical physicist who made foundational contributions to understanding atomic structure and old quantum theory, quantum theory, for which he received the No ...
, Lise Meitner
Elise Lise Meitner ( ; ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish nuclear physicist who was instrumental in the discovery of nuclear fission.
After completing her doctoral research in 1906, Meitner became the second woman ...
, Ernest Lawrence, Fermi, Chadwick, and others. As posed by Chadwick in his Bakerian Lecture in 1933, the primary question was the mass of the neutron relative to the proton. If the neutron's mass was less than the combined masses of a proton and an electron (), then the neutron could be a proton-electron composite because of the mass defect from the nuclear binding energy
Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is alwa ...
. If greater than the combined masses, then the neutron was elementary like the proton. The question was challenging to answer because the electron's mass is only 0.05% of the proton's, hence exceptionally precise measurements were required.
The difficulty of making the measurement is illustrated by the wide-ranging values for the mass of the neutron obtained from 1932 to 1934. The accepted value today is . In Chadwick's 1932 paper reporting on the discovery, he estimated the mass of the neutron to be between and . By bombarding boron with alpha particles, Frédéric and Irène Joliot-Curie obtained a high value of , while Ernest Lawrence's team at the University of California measured the small value using their new cyclotron
A cyclotron is a type of particle accelerator invented by Ernest Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Januar ...
.
In 1935 Chadwick and his doctoral student Maurice Goldhaber resolved the issue by reporting the first accurate measurement of the mass of the neutron. They used the 2.6 MeV gamma rays of Thallium
Thallium is a chemical element; it has Symbol (chemistry), symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Che ...
-208 (208Tl) (then known as thorium C") to photodisintegrate the deuteron.
:
In this reaction, the resulting proton and neutron have about equal kinetic energy, since their masses are about equal. The kinetic energy of the resulting proton could be measured (0.24 MeV), and therefore the deuteron's binding energy could be determined (2.6 MeV − 2(0.24 MeV) = 2.1 MeV, or ). The neutron's mass could then be determined by the simple mass balance
:
where ''m''d,p,n refer to the deuteron, proton, or neutron mass, and "b.e." is the binding energy. The masses of the deuteron and proton were known; Chadwick and Goldhaber used values 2.0142 Da and 1.0081 Da, respectively. They found that the neutron's mass was slightly greater than the mass of the proton or , depending on the precise value used for the deuteron mass. The mass of the neutron was too large to be a proton–electron composite, and the neutron was therefore identified as an elementary particle. Chadwick and Goldhaber predicted that a free neutron would be able to decay into a proton, electron, and neutrino (beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which an atomic nucleus emits a beta particle (fast energetic electron or positron), transforming into an isobar of that nuclide. For example, beta decay of a neutron ...
).
Neutron physics in the 1930s
Soon after the discovery of the neutron, indirect evidence suggested the neutron had an unexpected non-zero value for its magnetic moment. Attempts to measure the neutron's magnetic moment originated with the discovery by Otto Stern in 1933 inHamburg
Hamburg (, ; ), officially the Free and Hanseatic City of Hamburg,. is the List of cities in Germany by population, second-largest city in Germany after Berlin and List of cities in the European Union by population within city limits, 7th-lar ...
that the proton had an anomalously large magnetic moment. By 1934 groups led by Stern, now in Pittsburgh
Pittsburgh ( ) is a city in Allegheny County, Pennsylvania, United States, and its county seat. It is the List of municipalities in Pennsylvania#Municipalities, second-most populous city in Pennsylvania (after Philadelphia) and the List of Un ...
, and I. I. Rabi in New York had independently deduced that the magnetic moment of the neutron was negative and unexpectedly large by measuring the magnetic moments of the proton and deuteron
Deuterium (hydrogen-2, symbol H or D, also known as heavy hydrogen) is one of two Stable isotope ratio, stable isotopes of hydrogen; the other is protium, or hydrogen-1, H. The deuterium atomic nucleus, nucleus (deuteron) contains one proton and ...
. Values for the magnetic moment of the neutron were also determined by Robert Bacher (1933) at Ann Arbor
Ann Arbor is a city in Washtenaw County, Michigan, United States, and its county seat. The 2020 United States census, 2020 census recorded its population to be 123,851, making it the List of municipalities in Michigan, fifth-most populous cit ...
and I.Y. Tamm and S.A. Altshuler (1934) in the Soviet Union
The Union of Soviet Socialist Republics. (USSR), commonly known as the Soviet Union, was a List of former transcontinental countries#Since 1700, transcontinental country that spanned much of Eurasia from 1922 until Dissolution of the Soviet ...
from studies of the hyperfine structure of atomic spectra. By the late 1930s accurate values for the magnetic moment of the neutron had been deduced by the Rabi group using measurements employing newly developed nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
techniques. The large value for the proton's magnetic moment and the inferred negative value for the neutron's magnetic moment were unexpected and raised many questions.
 The discovery of the neutron immediately gave scientists a new tool for probing the properties of atomic nuclei. Alpha particles had been used over the previous decades in scattering experiments, but such particles, which are helium nuclei, have +2 charge. This charge makes it difficult for alpha particles to overcome the Coulomb repulsive force and interact directly with the nuclei of atoms. Since neutrons have no electric charge, they do not have to overcome this force to interact with nuclei. Almost coincident with their discovery, neutrons were used by Norman Feather, Chadwick's colleague and protege, in scattering experiments with nitrogen. Feather was able to show that neutrons interacting with nitrogen nuclei scattered to protons or induced nitrogen to disintegrate to form
The discovery of the neutron immediately gave scientists a new tool for probing the properties of atomic nuclei. Alpha particles had been used over the previous decades in scattering experiments, but such particles, which are helium nuclei, have +2 charge. This charge makes it difficult for alpha particles to overcome the Coulomb repulsive force and interact directly with the nuclei of atoms. Since neutrons have no electric charge, they do not have to overcome this force to interact with nuclei. Almost coincident with their discovery, neutrons were used by Norman Feather, Chadwick's colleague and protege, in scattering experiments with nitrogen. Feather was able to show that neutrons interacting with nitrogen nuclei scattered to protons or induced nitrogen to disintegrate to form boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
with the emission of an alpha particle. Feather was therefore the first to show that neutrons produce nuclear disintegrations.
In Rome
Rome (Italian language, Italian and , ) is the capital city and most populated (municipality) of Italy. It is also the administrative centre of the Lazio Regions of Italy, region and of the Metropolitan City of Rome. A special named with 2, ...
, Enrico Fermi and his team bombarded heavier elements with neutrons and found the products to be radioactive. By 1934 they had used neutrons to induce radioactivity in 22 different elements, many of these elements of high atomic number. Noticing that other experiments with neutrons at his laboratory seemed to work better on a wooden table than a marble table, Fermi suspected that the protons of the wood were slowing the neutrons and so increasing the chance for the neutron to interact with nuclei. Fermi therefore passed neutrons through paraffin wax to slow them and found that the radioactivity of some bombarded elements increased by a factor of tens to hundreds. The cross section for interaction with nuclei is much larger for slow neutrons than for fast neutrons. In 1938 Fermi received the Nobel Prize in Physics ''"for his demonstrations of the existence of new radioactive elements produced by neutron irradiation, and for his related discovery of nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a t ...
s brought about by slow neutrons"''. Later, Fermi recounted to Chandrasekhar that he was originally planning to put a piece of lead there, but an inexplicable, intuitive feeling made him put a paraffin in the spot instead.

Berlin
Berlin ( ; ) is the Capital of Germany, capital and largest city of Germany, by both area and List of cities in Germany by population, population. With 3.7 million inhabitants, it has the List of cities in the European Union by population withi ...
, the collaboration of Lise Meitner
Elise Lise Meitner ( ; ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish nuclear physicist who was instrumental in the discovery of nuclear fission.
After completing her doctoral research in 1906, Meitner became the second woman ...
and Otto Hahn
Otto Hahn (; 8 March 1879 – 28 July 1968) was a German chemist who was a pioneer in the field of radiochemistry. He is referred to as the father of nuclear chemistry and discoverer of nuclear fission, the science behind nuclear reactors and ...
, together with their assistant Fritz Strassmann, furthered the research begun by Fermi and his team when they bombarded uranium with neutrons. Between 1934 and 1938, Hahn, Meitner, and Strassmann found a great number of radioactive transmutation products from these experiments, all of which they regarded as transuranic. Transuranic nuclides are those that have an atomic number greater than uranium (92), formed by neutron absorption; such nuclides are not naturally occurring. In July 1938, Meitner was forced to escape antisemitic persecution in Nazi Germany
Nazi Germany, officially known as the German Reich and later the Greater German Reich, was the German Reich, German state between 1933 and 1945, when Adolf Hitler and the Nazi Party controlled the country, transforming it into a Totalit ...
after the Anschluss
The (, or , ), also known as the (, ), was the annexation of the Federal State of Austria into Nazi Germany on 12 March 1938.
The idea of an (a united Austria and Germany that would form a "German Question, Greater Germany") arose after t ...
, and she was able to secure a new position in Sweden. The decisive experiment on 16–17 December 1938 (using a chemical process called "radium–barium–mesothorium fractionation
Fractionation is a separation process in which a certain quantity of a mixture (of gasses, solids, liquids, enzymes, or isotopes, or a suspension) is divided during a phase transition, into a number of smaller quantities (fractions) in which t ...
") produced puzzling results: what they had understood to be three isotopes of radium were instead consistently behaving as barium. Radium (atomic number 88) and barium (atomic number 56) are in the same chemical group. By January 1939 Hahn had concluded that what they had thought were transuranic nuclides were instead much lighter nuclides, such as barium, lanthanum, cerium
Cerium is a chemical element; it has Chemical symbol, symbol Ce and atomic number 58. It is a hardness, soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it ...
and light platinoids. Meitner and her nephew Otto Frisch immediately and correctly interpreted these observations as resulting from nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
, a term coined by Frisch.
Hahn and his collaborators had detected the splitting of uranium nuclei, made unstable by neutron absorption, into lighter elements. Meitner and Frisch also showed that the fission of each uranium atom would release about 200 MeV of energy. The discovery of fission electrified the global community of atomic physicists and the public. In their second publication on nuclear fission, Hahn and Strassmann predicted the existence and liberation of additional neutrons during the fission process. Frédéric Joliot and his team proved this phenomenon to be a chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that sys ...
in March 1939. In 1945 Hahn received the 1944 Nobel Prize in Chemistry ''"for his discovery of the fission of heavy atomic nuclei".''
After 1939
 The discovery of nuclear fission at the end of 1938 marked a shift in the centers of nuclear research from
The discovery of nuclear fission at the end of 1938 marked a shift in the centers of nuclear research from Europe
Europe is a continent located entirely in the Northern Hemisphere and mostly in the Eastern Hemisphere. It is bordered by the Arctic Ocean to the north, the Atlantic Ocean to the west, the Mediterranean Sea to the south, and Asia to the east ...
to the United States. Large numbers of scientists were migrating to the United States to escape the troubles and antisemitism
Antisemitism or Jew-hatred is hostility to, prejudice towards, or discrimination against Jews. A person who harbours it is called an antisemite. Whether antisemitism is considered a form of racism depends on the school of thought. Antisemi ...
in Europe and the looming war (See Jewish scientists and the Manhattan Project). The new centers of nuclear research were the universities in the United States, particularly Columbia University
Columbia University in the City of New York, commonly referred to as Columbia University, is a Private university, private Ivy League research university in New York City. Established in 1754 as King's College on the grounds of Trinity Churc ...
in New York and the University of Chicago
The University of Chicago (UChicago, Chicago, or UChi) is a Private university, private research university in Chicago, Illinois, United States. Its main campus is in the Hyde Park, Chicago, Hyde Park neighborhood on Chicago's South Side, Chic ...
where Enrico Fermi had relocated, and a secret research facility at Los Alamos, New Mexico
New Mexico is a state in the Southwestern United States, Southwestern region of the United States. It is one of the Mountain States of the southern Rocky Mountains, sharing the Four Corners region with Utah, Colorado, and Arizona. It also ...
, established in 1942, the new home of the Manhattan project
The Manhattan Project was a research and development program undertaken during World War II to produce the first nuclear weapons. It was led by the United States in collaboration with the United Kingdom and Canada.
From 1942 to 1946, the ...
. This wartime project was focussed on the construction of nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission or atomic bomb) or a combination of fission and fusion reactions (thermonuclear weapon), producing a nuclear exp ...
s, exploiting the enormous energy released by the fission of uranium or plutonium
Plutonium is a chemical element; it has symbol Pu and atomic number 94. It is a silvery-gray actinide metal that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhibits six allotropes and four ...
through neutron-based chain reactions.
The discoveries of the neutron and positron in 1932 were the start of the discoveries of many new particles. Muon
A muon ( ; from the Greek letter mu (μ) used to represent it) is an elementary particle similar to the electron, with an electric charge of −1 '' e'' and a spin of ''ħ'', but with a much greater mass. It is classified as a ...
s were discovered in 1936. Pion
In particle physics, a pion (, ) or pi meson, denoted with the Greek alphabet, Greek letter pi (letter), pi (), is any of three subatomic particles: , , and . Each pion consists of a quark and an antiquark and is therefore a meson. Pions are the ...
s and kaons were discovered in 1947, while lambda particles were discovered in 1950. Throughout the 1950s and 1960s, a large number of particles called hadron
In particle physics, a hadron is a composite subatomic particle made of two or more quarks held together by the strong nuclear force. Pronounced , the name is derived . They are analogous to molecules, which are held together by the electri ...
s were discovered. A classification scheme for organizing all these particles, proposed independently by Murray Gell-Mann
Murray Gell-Mann (; September 15, 1929 – May 24, 2019) was an American theoretical physicist who played a preeminent role in the development of the theory of elementary particles. Gell-Mann introduced the concept of quarks as the funda ...
and
George Zweig
George Zweig (; born May 30, 1937) is an American physicist of Russian-Jewish origin. He was trained as a particle physicist under Richard Feynman. He introduced, independently of Murray Gell-Mann, the quark model (although he named it "aces"). ...
in 1964, became known as the quark model
In particle physics, the quark model is a classification scheme for hadrons in terms of their valence quarks—the quarks and antiquarks that give rise to the quantum numbers of the hadrons. The quark model underlies "flavor SU(3)", or the Eig ...
. By this model, particles such as the proton and neutron were not elementary, but composed of various configurations of a small number of other truly elementary particles called partons or quark
A quark () is a type of elementary particle and a fundamental constituent of matter. Quarks combine to form composite particles called hadrons, the most stable of which are protons and neutrons, the components of atomic nucleus, atomic nuclei ...
s. The quark model received experimental verification beginning in the late 1960s and finally provided an explanation for the neutron's anomalous magnetic moment.
Videos
Ernest Rutherford summarizes the state of nuclear physics in 1935.
(7 min., Nobelprize.org)
Hans Bethe discusses Chadwick and Goldhaber's work on deuteron disintegration.
(2 min., Web of Stories)
Explanatory notes
References
Further reading
Annotated bibliography for neutrons from the Alsos Digital Library for Nuclear Issues
* Abraham Pais, ''Inward Bound'', Oxford: Oxford University Press, 1986. . *
Herwig Schopper
Herwig Franz Schopper (born 28 February 1924) is a German experimental physicist. He was the director general of CERN from 1981 to 1988.
Biography
Schopper was born in Lanškroun, Czechoslovakia, to a family of Austrian descent. He obtained hi ...
, ''Weak interactions and nuclear beta decay'', Publisher, North-Holland Pub. Co., 1966.
* Ruth Lewin Sime, ''Lise Meitner: A Life in Physics'', Berkeley, University of California Press, 1996. .
* Roger H. Stuewer, "The Nuclear Electron Hypothesis". In ''Otto Hahn and the Rise of Nuclear Physics'', William R. Shea, ed. Dordrecht, Holland: D. Riedel Publishing Company. pp. 19–67, 1983. .
* Sin-Itiro Tomonaga, ''The Story of Spin'', The University of Chicago Press, 1997. {{ISBN, 9780226807942
Neutron
History of physics