Diamond cubic on:
[Wikipedia]
[Google]
[Amazon]


 The diamond cubic
The diamond cubic
 Diamond's cubic structure is in the Fdm
Diamond's cubic structure is in the Fdm
 Similarly,
Similarly,
/ref> though structures composed of skeletal
Software
to construct self-avoiding random walks on the diamond cubic lattice Crystal structure types Crystallography Cubes Infinite graphs Lattice points Minerals in space group 227 Regular graphs Articles containing video clips


 The diamond cubic
The diamond cubic crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pattern ...
is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, b ...
, other elements in group 14 also adopt this structure, including α-tin, the semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way ...
s silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
and germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors ...
, and silicon–germanium
SiGe ( or ), or silicon–germanium, is an alloy with any molar ratio of silicon and germanium, i.e. with a molecular formula of the form Si1−''x''Ge''x''. It is commonly used as a semiconductor material in integrated circuits (ICs) for heter ...
alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductili ...
s in any proportion. There are also crystals, such as the high-temperature form of cristobalite
Cristobalite is a mineral polymorph of silica that is formed at very high temperatures. It has the same chemical formula as quartz, SiO2, but a distinct crystal structure. Both quartz and cristobalite are polymorphs with all the members of the ...
, which have a similar structure, with one kind of atom (such as silicon in cristobalite) at the positions of carbon atoms in diamond but with another kind of atom (such as oxygen) halfway between those (see :Minerals in space group 227).
Although often called the diamond lattice, this structure is not a lattice in the technical sense of this word used in mathematics.
Crystallographic structure
space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it uncha ...
(space group 227), which follows the face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
Bravais lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n ...
. The lattice describes the repeat pattern; for diamond cubic crystals this lattice is "decorated" with a ''motif'' of two tetrahedrally bonded atoms in each primitive cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessari ...
, separated by of the width of the unit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessaril ...
in each dimension. The diamond lattice can be viewed as a pair of intersecting face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
lattices, with each separated by of the width of the unit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessaril ...
in each dimension. Many compound semiconductor
Semiconductor materials are nominally small band gap insulators. The defining property of a semiconductor material is that it can be compromised by doping it with impurities that alter its electronic properties in a controllable way.
Because of ...
s such as gallium arsenide
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a zinc blende crystal structure.
Gallium arsenide is used in the manufacture of devices such as microwave frequency integrated circuits, monolithic microwave integrated c ...
, β-silicon carbide
Silicon carbide (SiC), also known as carborundum (), is a hard chemical compound containing silicon and carbon. A semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder and crystal s ...
, and indium antimonide adopt the analogous zincblende structure
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties ...
, where each atom has nearest neighbors of an unlike element. Zincblende's space group is F3m, but many of its structural properties are quite similar to the diamond structure.
The atomic packing factor In crystallography, atomic packing factor (APF), packing efficiency, or packing fraction is the fraction of volume in a crystal structure that is occupied by constituent particles. It is a dimensionless quantity and always less than unity. In atomi ...
of the diamond cubic structure (the proportion of space that would be filled by spheres that are centered on the vertices of the structure and are as large as possible without overlapping) is ≈ 0.34, significantly smaller (indicating a less dense structure) than the packing factors for the face-centered and body-centered cubic lattices. Zincblende structures have higher packing factors than 0.34 depending on the relative sizes of their two component atoms.
The first-, second-, third-, fourth-, and fifth-nearest-neighbor distances in units of the cubic lattice constant are , , , 1 and , respectively.
Mathematical structure
Mathematically, the points of the diamond cubic structure can be given coordinates as a subset of a three-dimensional integer lattice by using a cubic unit cell four units across. With these coordinates, the points of the structure have coordinates (''x'', ''y'', ''z'') satisfying the equations :''x'' = ''y'' = ''z'' (mod 2), and :''x'' + ''y'' + ''z'' = 0 or 1 (mod 4).. There are eight points (modulo 4) that satisfy these conditions: :(0,0,0), (0,2,2), (2,0,2), (2,2,0), :(3,3,3), (3,1,1), (1,3,1), (1,1,3) All of the other points in the structure may be obtained by adding multiples of four to the ''x'', ''y'', and ''z'' coordinates of these eight points. Adjacent points in this structure are at distance apart in the integer lattice; the edges of the diamond structure lie along the body diagonals of the integer grid cubes. This structure may be scaled to a cubical unit cell that is some number ''a'' of units across by multiplying all coordinates by . Alternatively, each point of the diamond cubic structure may be given by four-dimensional integer coordinates whose sum is either zero or one. Two points are adjacent in the diamond structure if and only if their four-dimensional coordinates differ by one in a single coordinate. The total difference in coordinate values between any two points (their four-dimensionalManhattan distance
A taxicab geometry or a Manhattan geometry is a geometry whose usual distance function or metric of Euclidean geometry is replaced by a new metric in which the distance between two points is the sum of the absolute differences of their Cartesian co ...
) gives the number of edges in the shortest path
In graph theory, the shortest path problem is the problem of finding a path between two vertices (or nodes) in a graph such that the sum of the weights of its constituent edges is minimized.
The problem of finding the shortest path between ...
between them in the diamond structure. The four nearest neighbors of each point may be obtained, in this coordinate system, by adding one to each of the four coordinates, or by subtracting one from each of the four coordinates, accordingly as the coordinate sum is zero or one. These four-dimensional coordinates may be transformed into three-dimensional coordinates by the formula
:(''a'', ''b'', ''c'', ''d'') → (''a'' + ''b'' − ''c'' − ''d'', ''a'' − ''b'' + ''c'' − ''d'', −''a'' + ''b'' + ''c'' − ''d'')..
Because the diamond structure forms a distance-preserving subset of the four-dimensional integer lattice, it is a partial cube.
Yet another coordinatization of the diamond cubic involves the removal of some of the edges from a three-dimensional grid graph. In this coordinatization, which has a distorted geometry from the standard diamond cubic structure but has the same topological structure, the vertices of the diamond cubic are represented by all possible 3d grid points and the edges of the diamond cubic are represented by a subset of the 3d grid edges..
The diamond cubic is sometimes called the "diamond lattice" but it is not, mathematically, a lattice: there is no translational symmetry
In geometry, to translate a geometric figure is to move it from one place to another without rotating it. A translation "slides" a thing by .
In physics and mathematics, continuous translational symmetry is the invariance of a system of equati ...
that takes the point (0,0,0) into the point (3,3,3), for instance. However, it is still a highly symmetric structure: any incident pair of a vertex and edge can be transformed into any other incident pair by a congruence of Euclidean space
Euclidean space is the fundamental space of geometry, intended to represent physical space. Originally, that is, in Euclid's ''Elements'', it was the three-dimensional space of Euclidean geometry, but in modern mathematics there are Euclidea ...
. Moreover, the diamond crystal as a network in space has a strong isotropic property. Namely, for any two vertices ''x'' and ''y'' of the crystal net, and for any ordering of the edges adjacent to ''x'' and any ordering of the edges adjacent to ''y'', there is a net-preserving congruence taking ''x'' to ''y'' and each ''x''-edge to the similarly ordered ''y''-edge. Another (hypothetical) crystal with this property is the Laves graph
In geometry and crystallography, the Laves graph is an infinite and highly symmetric system of points and line segments in three-dimensional Euclidean space, forming a periodic graph. Three equal-length segments meet at 120° angles at each po ...
(also called the K4 crystal, (10,3)-a, or the diamond twin).
Mechanical properties
The compressive strength and hardness ofdiamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, b ...
and various other materials, such as boron nitride
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal ...
, (which has the closely related zincblende structure
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties ...
) is attributed to the diamond cubic structure.
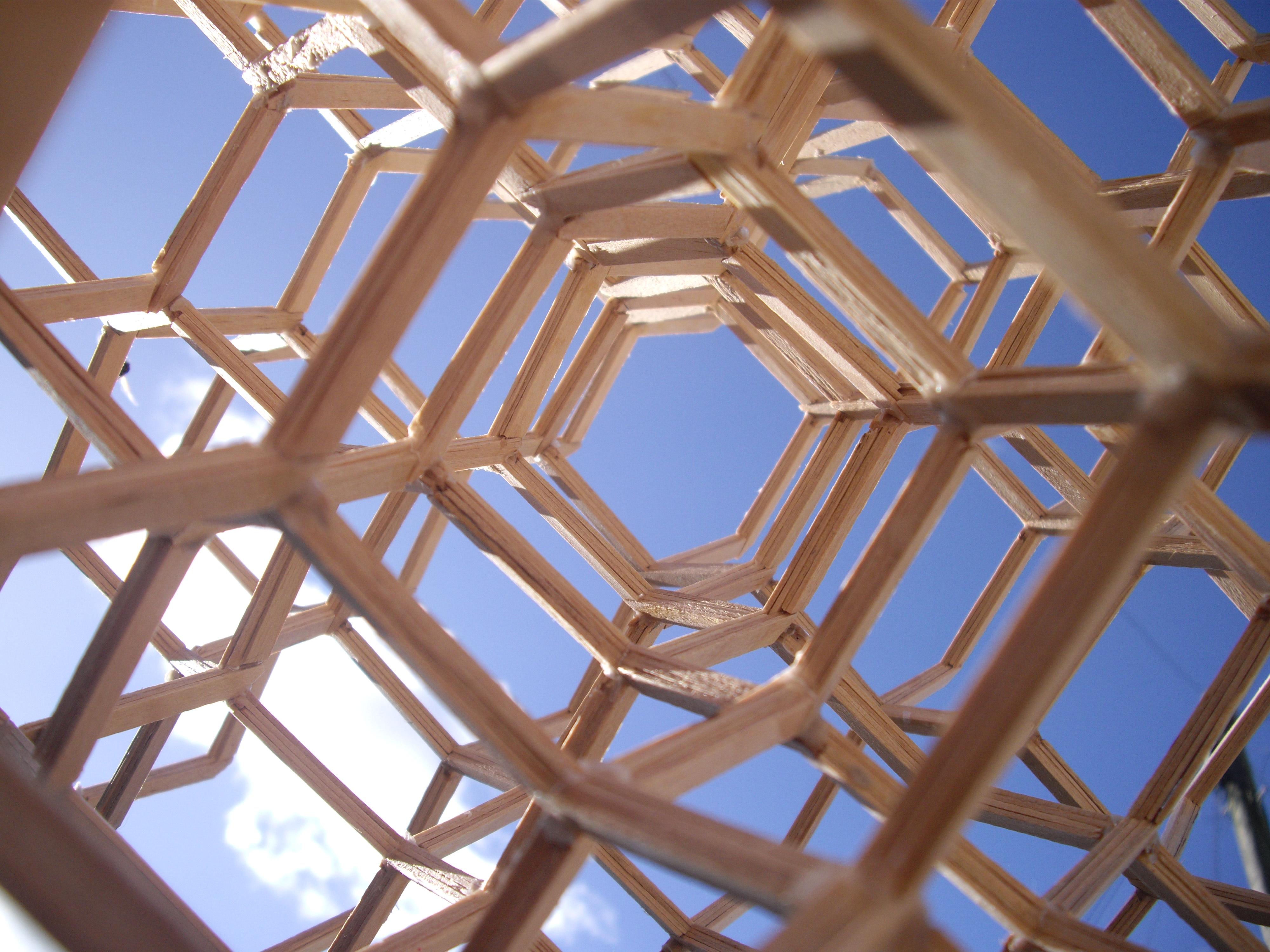
truss
A truss is an assembly of ''members'' such as beams, connected by ''nodes'', that creates a rigid structure.
In engineering, a truss is a structure that "consists of two-force members only, where the members are organized so that the assembl ...
systems that follow the diamond cubic geometry have a high capacity to withstand compression, by minimizing the unbraced length of individual strut
A strut is a structural component commonly found in engineering, aeronautics, architecture and anatomy. Struts generally work by resisting longitudinal compression, but they may also serve in tension.
Human anatomy
Part of the functionality o ...
s. The diamond cubic geometry has also been considered for the purpose of providing structural rigidity
In discrete geometry and mechanics, structural rigidity is a combinatorial theory for predicting the flexibility of ensembles formed by rigid bodies connected by flexible linkages or hinges.
Definitions
Rigidity is the property of a struct ...
Gilman, J. Tetrahedral Truss, USA, United States Patents, US4446666, 198/ref> though structures composed of skeletal
triangle
A triangle is a polygon with three edges and three vertices. It is one of the basic shapes in geometry. A triangle with vertices ''A'', ''B'', and ''C'' is denoted \triangle ABC.
In Euclidean geometry, any three points, when non- colline ...
s, such as the octet truss, have been found to be more effective for this purpose.
See also
* * * *References
External links
*{{Commons-inline, Diamond cubicSoftware
to construct self-avoiding random walks on the diamond cubic lattice Crystal structure types Crystallography Cubes Infinite graphs Lattice points Minerals in space group 227 Regular graphs Articles containing video clips