deoxygenation on:
[Wikipedia]
[Google]
[Amazon]
Deoxygenation is a
Link
/ref> For example, in the deoxygenation of the
chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breakin ...
involving the removal of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less comm ...
and gas purifiers. As applied to organic compounds, deoxygenation is a component of fuels production as well a type of reaction employed in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
, e.g. of pharmaceuticals
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and r ...
.
Deoxygenation of C-O bonds
With replacement by H2
The main examples involving the replacement of an oxo group by two hydrogen atoms (A=O → AH2) arehydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon– heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. S ...
. Typical examples use metal catalysts and H2 as the reagent. Conditions are typically more forcing than hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic c ...
.
Stoichiometric reactions that effect deoxygenation include the Wolff–Kishner reduction
The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has se ...
for aryl ketones. The replacement of a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
group by hydrogen (A-OH → A-H) is the point of the Barton–McCombie deoxygenation
The Barton–McCombie deoxygenation is an organic reaction in which a hydroxy functional group in an organic compound is replaced by a hydrogen to give an alkyl group. It is named after British chemists Sir Derek Harold Richard Barton and Stuart ...
and the Markó–Lam deoxygenation The Markó–Lam deoxygenation is an organic chemistry reaction where the hydroxy functional group in an organic compound is replaced by a hydrogen atom to give an alkyl group. The Markó-Lam reaction is a variant of the Bouveault–Blanc reduction ...
.
Biomass valorization
Deoxygenation is an important goal of the conversion of biomass to useful fuels and chemicals. Partial deoxygenation is effected bydehydration
In physiology, dehydration is a lack of total body water, with an accompanying disruption of metabolic processes. It occurs when free water loss exceeds free water intake, usually due to exercise, disease, or high environmental temperature. Mi ...
and decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is ...
.
Other routes
Oxygen groups can also be removed by the reductive coupling of ketones, as illustrated by theMcMurry reaction
The McMurry reaction is an organic reaction in which two ketone or aldehyde groups are coupled to form an alkene using a titanium chloride compound such as titanium(III) chloride and a reducing agent. The reaction is named after its co-discoverer, ...
.
:
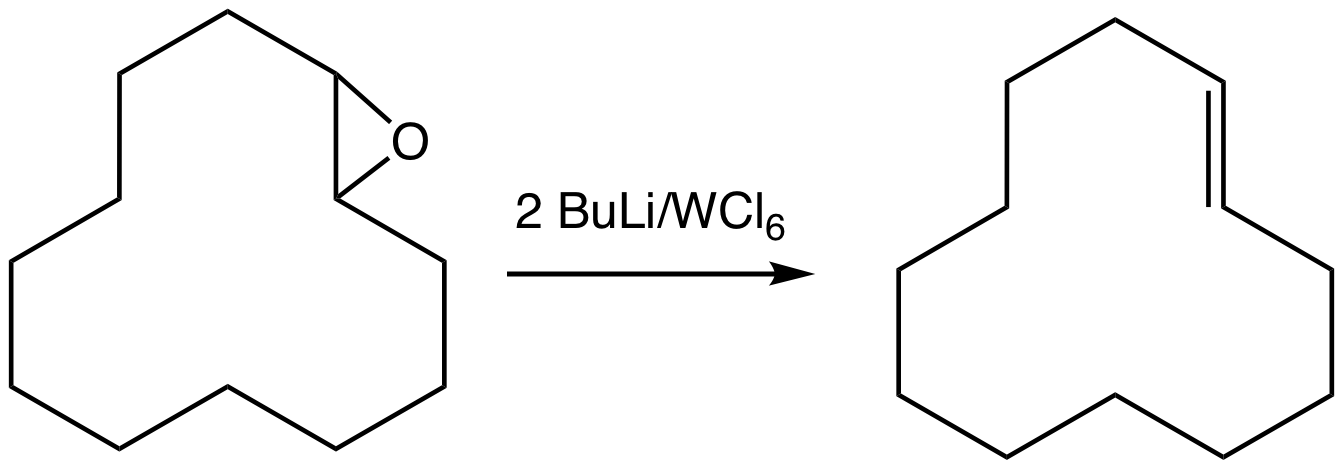
Epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale f ...
s can be deoxygenated using the oxophilic reagent produced by combining tungsten hexachloride
Tungsten hexachloride is the chemical compound of tungsten and chlorine with the formula WCl6. This dark violet blue species exists as a volatile solid under standard conditions. It is an important starting reagent in the preparation of tungsten ...
and ''n''-butyllithium generates the alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
. This reaction can proceed with loss or retention of configuration.
:
Deoxygenation of S-O and P-O bonds
N=O bonds
Nitroaromatics are deoxygenated by strongly reducing silyl reagents such as N,N'-bis(trimethylsilyl)-4,4'-bipyridinylidene.P=O bonds
Phosphorus occurs in nature as oxides, so to produce elemental form of the element, deoxygenation is required. The main method involvescarbothermic reduction
Carbothermic reactions involve the reduction of substances, often metal oxides (O^2-), using carbon as the reducing agent. These chemical reactions are usually conducted at temperatures of several hundred degrees Celsius. Such processes are applie ...
(i.e., carbon is the deoxygenation agent).
:4 Ca5(PO4)3F + 18 SiO2 + 30 C → 3 P4 + 30 CO + 18 CaSiO3 + 2 CaF2
Oxophilic
Oxophilicity is the tendency of certain chemical compounds to form oxides by hydrolysis or abstraction of an oxygen atom from another molecule, often from organic compounds. The term is often used to describe metal centers, commonly the early trans ...
main group compounds are useful reagents for certain deoxygenations conducted on laboratory scale. The highly oxophilic reagent hexachlorodisilane (Si2Cl6) stereospecifically deoxygenates phosphine oxide
Phosphine oxides are phosphorus compounds with the formula OPX3. When X = alkyl or aryl, these are organophosphine oxides. Triphenylphosphine oxide is an example. An inorganic phosphine oxide is phosphoryl chloride (POCl3).
Structure and bonding ...
s.
S=O bonds
A chemical reagent for the deoxygenation of many sulfur and nitrogen oxo compounds is the combination trifluoroacetic anhydride/sodium iodide
Sodium iodide ( chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anion ...
.''Trifluoroacetic anhydride-sodium iodide reagent. Nature and applications'' Arkivoc
''Arkivoc'' (''Archive for Organic Chemistry'') is a peer-reviewed open access scientific journal covering all aspects of organic chemistry. It is published by the non-profit organization Arkat USA, which was established in 2000 through a personal ...
2007 (JE-2136MR) Zbigniew H. Kudzin, Marcin H. Kudzin, Józef Drabowicz, and Andrzej KotyńskLink
/ref> For example, in the deoxygenation of the
sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. ...
''diphenylsulfoxide'' to the sulfide
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to chemical compounds lar ...
''diphenylsulfide'':
:
The reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
is based on the activation of the sulfoxide by a trifluoroacetyl group and oxidation of iodine. Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vio ...
is formed quantitatively in this reaction and therefore the reagent is used for the analytical detection of many oxo compounds.
:
See also
* Degassing * Preparation of stable carbenes *Ocean deoxygenation
Ocean deoxygenation is the reduction of the oxygen content of the global oceans and coastal zones due to human activities as a consequence of anthropogenic emissions of carbon dioxide and eutrophication-driven excess production. It is manife ...
*Oxophilicity
Oxophilicity is the tendency of certain chemical compounds to form oxides by hydrolysis or abstraction of an oxygen atom from another molecule, often from organic compounds. The term is often used to describe metal centers, commonly the early trans ...
References
{{Reflist Organic redox reactions Gas technologies