Contact electrification on:
[Wikipedia]
[Google]
[Amazon]
Contact electrification is a phrase that describes a phenomenon whereby surfaces become electrically charged, via a number of possible mechanisms, when two or more objects come within close proximity of one another. When two objects are "touched" together, sometimes the objects become spontaneously charged. One object may develop a net negative charge, while the other develops an equal and opposite positive charge. This effect may be caused by various physical processes – triboelectricity, the Volta effect, differing

work function
In solid-state physics, the work function (sometimes spelt workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" ...
s of metals, and others which are collective referred to as contact electrification.
The contact electrification phenomenon allowed the construction of so-called 'frictional' electrostatic generator
An electrostatic generator, or electrostatic machine, is an electrical generator that produces ''static electricity'', or electricity at high voltage and low continuous current. The knowledge of static electricity dates back to the earliest ci ...
s such as Ramsden's or Winter's machines, but it also led directly to the development of useful devices such as batteries, fuel cells, electroplating, thermocouples. Contact between materials is responsible for such modern electrical technology as semiconductor junction devices including radio detector diodes, photocell
Photodetectors, also called photosensors, are sensors of light or other electromagnetic radiation. There is a wide variety of photodetectors which may be classified by mechanism of detection, such as photoelectric or photochemical effects, or b ...
s, LEDs, and thermoelectric cells.
History
The theory held that static electricity was generated by means of contact between dissimilar materials, and was in close agreement with the principles of static electricity as then understood. It was eventually replaced by the current theory ofelectrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
, namely, that electricity is generated by the action of chemistry and the exchange of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
s between atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
s making up the battery. An important fact leading to the rejection of the theory of contact tension was the observation that corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
, that is, the chemical degradation of the battery, seemed unavoidable with its use, and that the more electricity was drawn from the battery, the faster the corrosion proceeded.
The Volta effect (described below) corresponds to a weak electric potential difference developed by the contact of different metals. Nowadays, this is often known as a contact potential difference. This effect was first discovered by Alessandro Volta, and can be measured using a capacitance electroscope comprising different metals. However, this effect does not, by itself, account for the action of electric batteries.
A number of high voltage
High voltage electricity refers to electrical potential large enough to cause injury or damage. In certain industries, ''high voltage'' refers to voltage above a certain threshold. Equipment and conductors that carry high voltage warrant sp ...
dry piles were invented between the early 19th century and the 1830s in an attempt to determine the answer to this question, and specifically to support Volta’s hypothesis of contact tension. The Oxford Electric Bell
The Oxford Electric Bell or Clarendon Dry Pile is an experimental electric bell, in particular a type of bell that uses the electrostatic clock principle that was set up in 1840 and which has run nearly continuously ever since. It was one of th ...
is one example. Francis Ronalds
Sir Francis Ronalds FRS (21 February 17888 August 1873) was an English scientist and inventor, and arguably the first electrical engineer. He was knighted for creating the first working electric telegraph over a substantial distance. In 1816 ...
in 1814 was one of the first to realise that dry piles also worked through chemical reaction rather than metal to metal contact, even though corrosion was not visible due to the very small currents generated.
Triboelectric contact
If two different insulators are touched together, such as when a piece of rubber is touched against a piece of glass, then the surface of the rubber will acquire an excess negative charge, and the glass will acquire an equal positive charge. If the surfaces are then pulled apart, a veryhigh voltage
High voltage electricity refers to electrical potential large enough to cause injury or damage. In certain industries, ''high voltage'' refers to voltage above a certain threshold. Equipment and conductors that carry high voltage warrant sp ...
is produced. This so-called "tribo" or "rubbing" effect is not well understood. It may be caused by electron-stealing via quantum tunneling
In physics, a quantum (plural quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a physical property can be "quantized" is referred to as "the hypothesis of quantizati ...
, or by transfer of surface ions. Friction is not required, although in many situations it greatly increases the phenomenon. Certain phenomena related to frictionally generated electrostatic charges have been known since antiquity, though of course the modern theory of electricity was developed after the Scientific Revolution
The Scientific Revolution was a series of events that marked the emergence of modern science during the early modern period, when developments in mathematics, physics, astronomy, biology (including human anatomy) and chemistry transfo ...
.
Solid-solid contact
The mechanism of contact electrification (CE) between solid-solid has been debated for more than 2600 years. A most controversial topic in CE is the identity of the charge carriers: electron transfer, ion transfer or even materials species transfer. Recent studies by using Kelvin probe force microscopy suggest that electron transfer is the dominating charge carrier in CE for solid-solid cases. When the interatomic distance between two atoms belonging to two materials is shorter than the normal bonding length (typically ~0.2 nm), the electrons will transfer at the interface. It implies that a strong electron cloud overlap (or wave function overlap) between the two atoms/molecules in the repulsive region will reduce the interatomic potential barrier (Fig. 1), and result in electron transition between the atoms/molecules. The contact/friction force in CE is to induce strong overlap between the electron clouds (or wave function in physics, bonding in chemistry).
Liquid-solid contact
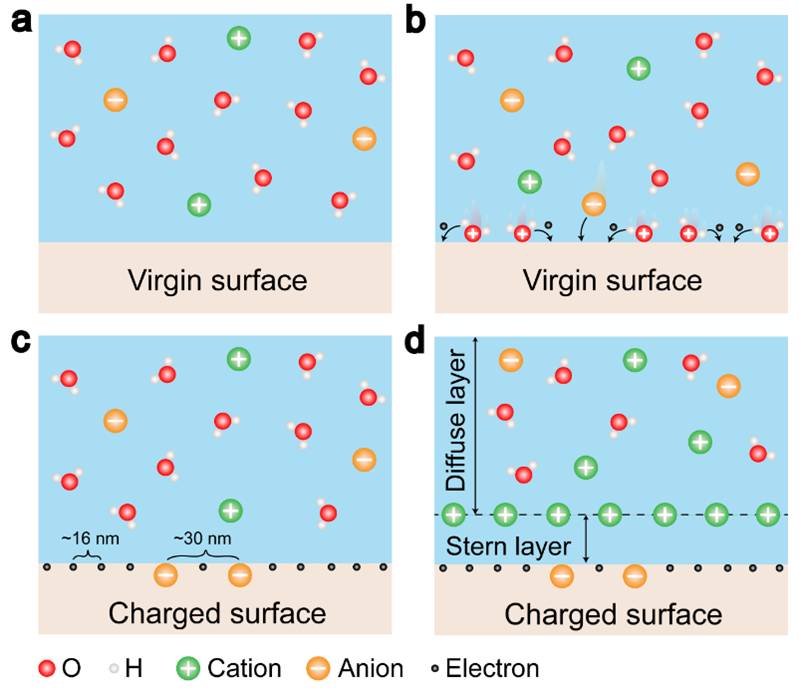
Besides ion transfer at liquid-solid interface, electron transfer occurs as well. As for the liquid-solid case, molecules in the liquid would have electron cloud overlap with the atoms on the solid surface at the very first contact with a virginal solid surface, and electron transfer is required in order to create the first layer of electrostatic charges on the solid surface. Then, ion transfer is the second step, which is a redistribution of the ions in solution considering electrostatic interactions with the charged solid surface (Fig. 2). Both electron transfer and ion transfer co-exist at liquid-solid interface.
Electrolytic-metallic contact
If a piece of metal is touched against an electrolytic material, the metal will spontaneously become charged, while the electrolyte will acquire an equal and opposite charge. Upon first contact, a chemical reaction called a ' half-cell reaction' occurs on the metal surface. As metal ions are transferred to or from the electrolyte, and as the metal and electrolyte become oppositely charged, the increasing voltage at the thin insulating layer between metal and electrolyte will oppose the motion of the flowing ions, causing the chemical reaction to come to a stop. If a second piece of a different type of metal is placed in the same electrolyte bath, it will charge up and rise to a different voltage. If the first metal piece is touched against the second, the voltage on the two metal pieces will be forced closer together, and the chemical reactions will run constantly. In this way the 'contact electrification' becomes continuous. At the same time, an electric current will appear, with the path forming a closed loop which leads from one metal part to the other, through the chemical reactions on the first metal surface, through the electrolyte, then back through the chemical reactions on the second metal surface. In this way, contact electrification leads to the invention of theGalvanic cell
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous Oxidation-Reduction reactions. A common apparatus ...
or battery.
Metallic contact
If two metals having differingwork function
In solid-state physics, the work function (sometimes spelt workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" ...
s are touched together, one steals electrons from the other, and the opposite net charges grow larger and larger; this is the Volta effect. The process is halted when the difference in electric potential (electrostatic potential) between the two metals reaches a particular value, namely the difference in work function values - usually less than one volt. At this point, the Fermi levels for the two metals are equal, and there is no voltage difference between them. f there were a voltage difference between them, then a current would flow between them: so "zero current" implies "zero voltage difference".Semiconductor contact
If a metal touches a semiconductor material, or if two different semiconductors are placed into contact, one becomes charged slightly positive and the other slightly negative. It is found that if this junction between semiconductors is connected to a power supply, and if the power supply is set to a voltage slightly higher than the natural voltage appearing because of contact electrification, then for one polarity of voltage there will be a current between the two semiconductor parts, but if the polarity is reversed, the current stops. Thus contact between materials lead to the invention of the semiconductor diode or rectifier and triggered the revolution insemiconductor electronics
A semiconductor device is an electronic component that relies on the electronic properties of a semiconductor material (primarily silicon, germanium, and gallium arsenide, as well as organic semiconductors) for its function. Its conductivity li ...
and physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which r ...
.
In materials with a direct band gap
In semiconductor physics, the band gap of a semiconductor can be of two basic types, a direct band gap or an indirect band gap. The minimal-energy state in the conduction band and the maximal-energy state in the valence band are each characteriz ...
, if bright light is aimed at one part of the contact area between the two semiconductors, the voltage at that spot will rise, and an electric current will appear. When considering light in the context of contact electrification{{what, date=April 2014, the light energy is changed directly into electrical energy, allowing creation of solar cells. Later it was found that the same process can be reversed, and if a current is forced backwards across the contact region between the semiconductors, sometimes light will be emitted, allowing creation of the light-emitting diode
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (co ...
(LED).
References